User:Bci2/Books/Wk4
| The Wikimedia Foundation's book rendering service has been withdrawn. Please upload your Wikipedia book to one of the external rendering services. |
You can still create and edit a book design using the Book Creator and upload it to an external rendering service:
|
| This user book is a user-generated collection of Wikipedia articles that can be easily saved, rendered electronically, and ordered as a printed book. If you are the creator of this book and need help, see Help:Books (general tips) and WikiProject Wikipedia-Books (questions and assistance). Edit this book: Book Creator · Wikitext Order a printed copy from: PediaPress [ About ] [ Advanced ] [ FAQ ] [ Feedback ] [ Help ] [ WikiProject ] [ Recent Changes ] | ||||||||
WildBot has found potential problems with this book. Please check the talk page for a detailed explanation of what these might be and how to fix them. When these are resolved, you can remove |wildbot=yes from this template. |
Wk4vol1: Advanced DNA Modelling
[edit]DNA
[edit]
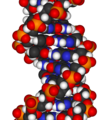
Deoxyribonucleic acid (DNA) is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms and some viruses. The main role of DNA molecules is the long-term storage of information. DNA is often compared to a set of blueprints or a recipe, or a code, since it contains the instructions needed to construct other components of cells, such as proteins and RNA molecules. The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in regulating the use of this genetic information.
Chemically, DNA consists of two long polymers of simple units called nucleotides, with backbones made of sugars and phosphate groups joined by ester bonds. These two strands run in opposite directions to each other and are therefore anti-parallel. Attached to each sugar is one of four types of molecules called bases. It is the sequence of these four bases along the backbone that encodes information. This information is read using the genetic code, which specifies the sequence of the amino acids within proteins. The code is read by copying stretches of DNA into the related nucleic acid RNA, in a process called transcription.
Within cells, DNA is organized into structures called chromosomes. These chromosomes are duplicated before cells divide, in a process called DNA replication. Eukaryotic organisms (animals, plants, fungi, and protists) store most of their DNA inside the cell nucleus and some of their DNA in the mitochondria. Prokaryotes (bacteria and archaea) however, store their DNA in the cell's cytoplasm. Within the chromosomes, chromatin proteins such as histones compact and organize DNA. These compact structures guide the interactions between DNA and other proteins, helping control which parts of the DNA are transcribed.
Properties
[edit]
DNA is a long polymer made from repeating units called nucleotides.[1][2][3] The DNA chain is 22 to 26 Ångströms wide (2.2 to 2.6 nanometres), and one nucleotide unit is 3.3 Å (0.33 nm) long.[4] Although each individual repeating unit is very small, DNA polymers can be very large molecules containing millions of nucleotides. For instance, the largest human chromosome, chromosome number 1, is approximately 220 million base pairs long.[5]
In living organisms, DNA does not usually exist as a single molecule, but instead as a pair of molecules that are held tightly together.[6][7] These two long strands entwine like vines, in the shape of a double helix. The nucleotide repeats contain both the segment of the backbone of the molecule, which holds the chain together, and a base, which interacts with the other DNA strand in the helix. In general, a base linked to a sugar is called a nucleoside and a base linked to a sugar and one or more phosphate groups is called a nucleotide. If multiple nucleotides are linked together, as in DNA, this polymer is called a polynucleotide.[8]
The backbone of the DNA strand is made from alternating phosphate and sugar residues.[9] The sugar in DNA is 2-deoxyribose, which is a pentose (five-carbon) sugar. The sugars are joined together by phosphate groups that form phosphodiester bonds between the third and fifth carbon atoms of adjacent sugar rings. These asymmetric bonds mean a strand of DNA has a direction. In a double helix the direction of the nucleotides in one strand is opposite to their direction in the other strand. This arrangement of DNA strands is called antiparallel. The asymmetric ends of DNA strands are referred to as the 5′ (five prime) and 3′ (three prime) ends, with the 5' end being that with a terminal phosphate group and the 3' end that with a terminal hydroxyl group. One of the major differences between DNA and RNA is the sugar, with 2-deoxyribose being replaced by the alternative pentose sugar ribose in RNA.[7]
The DNA double helix is stabilized by hydrogen bonds between the bases attached to the two strands. The four bases found in DNA are adenine (abbreviated A), cytosine (C), guanine (G) and thymine (T). These four bases are attached to the sugar/phosphate to form the complete nucleotide, as shown for adenosine monophosphate.
These bases are classified into two types; adenine and guanine are fused five- and six-membered heterocyclic compounds called purines, while cytosine and thymine are six-membered rings called pyrimidines.[7] A fifth pyrimidine base, called uracil (U), usually takes the place of thymine in RNA and differs from thymine by lacking a methyl group on its ring. Uracil is not usually found in DNA, occurring only as a breakdown product of cytosine.
Grooves
[edit]
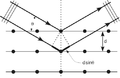
Twin helical strands form the DNA backbone. Another double helix may be found by tracing the spaces, or grooves, between the strands. These voids are adjacent to the base pairs and may provide a binding site. As the strands are not directly opposite each other, the grooves are unequally sized. One groove, the major groove, is 22 Å wide and the other, the minor groove, is 12 Å wide.[11] The narrowness of the minor groove means that the edges of the bases are more accessible in the major groove. As a result, proteins like transcription factors that can bind to specific sequences in double-stranded DNA usually make contacts to the sides of the bases exposed in the major groove.[12] This situation varies in unusual conformations of DNA within the cell (see below), but the major and minor grooves are always named to reflect the differences in size that would be seen if the DNA is twisted back into the ordinary B form.
Base pairing
[edit]Each type of base on one strand forms a bond with just one type of base on the other strand. This is called complementary base pairing. Here, purines form hydrogen bonds to pyrimidines, with A bonding only to T, and C bonding only to G. This arrangement of two nucleotides binding together across the double helix is called a base pair. As hydrogen bonds are not covalent, they can be broken and rejoined relatively easily. The two strands of DNA in a double helix can therefore be pulled apart like a zipper, either by a mechanical force or high temperature.[13] As a result of this complementarity, all the information in the double-stranded sequence of a DNA helix is duplicated on each strand, which is vital in DNA replication. Indeed, this reversible and specific interaction between complementary base pairs is critical for all the functions of DNA in living organisms.[2]

|

|
The two types of base pairs form different numbers of hydrogen bonds, AT forming two hydrogen bonds, and GC forming three hydrogen bonds (see figures, left). DNA with high GC-content is more stable than DNA with low GC-content, but contrary to popular belief, this is not due to the extra hydrogen bond of a GC basepair but rather the contribution of stacking interactions (hydrogen bonding merely provides specificity of the pairing, not stability).[14] As a result, it is both the percentage of GC base pairs and the overall length of a DNA double helix that determine the strength of the association between the two strands of DNA. Long DNA helices with a high GC content have stronger-interacting strands, while short helices with high AT content have weaker-interacting strands.[15] In biology, parts of the DNA double helix that need to separate easily, such as the TATAAT Pribnow box in some promoters, tend to have a high AT content, making the strands easier to pull apart.[16] In the laboratory, the strength of this interaction can be measured by finding the temperature required to break the hydrogen bonds, their melting temperature (also called Tm value). When all the base pairs in a DNA double helix melt, the strands separate and exist in solution as two entirely independent molecules. These single-stranded DNA molecules have no single common shape, but some conformations are more stable than others.[17]
Sense and antisense
[edit]A DNA sequence is called "sense" if its sequence is the same as that of a messenger RNA copy that is translated into protein.[18] The sequence on the opposite strand is called the "antisense" sequence. Both sense and antisense sequences can exist on different parts of the same strand of DNA (i.e. both strands contain both sense and antisense sequences). In both prokaryotes and eukaryotes, antisense RNA sequences are produced, but the functions of these RNAs are not entirely clear.[19] One proposal is that antisense RNAs are involved in regulating gene expression through RNA-RNA base pairing.[20]
A few DNA sequences in prokaryotes and eukaryotes, and more in plasmids and viruses, blur the distinction between sense and antisense strands by having overlapping genes.[21] In these cases, some DNA sequences do double duty, encoding one protein when read along one strand, and a second protein when read in the opposite direction along the other strand. In bacteria, this overlap may be involved in the regulation of gene transcription,[22] while in viruses, overlapping genes increase the amount of information that can be encoded within the small viral genome.[23]
Supercoiling
[edit]DNA can be twisted like a rope in a process called DNA supercoiling. With DNA in its "relaxed" state, a strand usually circles the axis of the double helix once every 10.4 base pairs, but if the DNA is twisted the strands become more tightly or more loosely wound.[24] If the DNA is twisted in the direction of the helix, this is positive supercoiling, and the bases are held more tightly together. If they are twisted in the opposite direction, this is negative supercoiling, and the bases come apart more easily. In nature, most DNA has slight negative supercoiling that is introduced by enzymes called topoisomerases.[25] These enzymes are also needed to relieve the twisting stresses introduced into DNA strands during processes such as transcription and DNA replication.[26]

Alternate DNA structures
[edit]
DNA exists in many possible conformations that include A-DNA, B-DNA, and Z-DNA forms, although, only B-DNA and Z-DNA have been directly observed in functional organisms.[9] The conformation that DNA adopts depends on the hydration level, DNA sequence, the amount and direction of supercoiling, chemical modifications of the bases, the type and concentration of metal ions, as well as the presence of polyamines in solution.[28]
The first published reports of A-DNA X-ray diffraction patterns— and also B-DNA used analyses based on Patterson transforms that provided only a limited amount of structural information for oriented fibers of DNA.[29][30] An alternate analysis was then proposed by Wilkins et al., in 1953, for the in vivo B-DNA X-ray diffraction/scattering patterns of highly hydrated DNA fibers in terms of squares of Bessel functions.[31] In the same journal, Watson and Crick presented their molecular modeling analysis of the DNA X-ray diffraction patterns to suggest that the structure was a double-helix.[6]
Although the `B-DNA form' is most common under the conditions found in cells,[32] it is not a well-defined conformation but a family of related DNA conformations[33] that occur at the high hydration levels present in living cells. Their corresponding X-ray diffraction and scattering patterns are characteristic of molecular paracrystals with a significant degree of disorder.[34][35]
Compared to B-DNA, the A-DNA form is a wider right-handed spiral, with a shallow, wide minor groove and a narrower, deeper major groove. The A form occurs under non-physiological conditions in partially dehydrated samples of DNA, while in the cell it may be produced in hybrid pairings of DNA and RNA strands, as well as in enzyme-DNA complexes.[36][37] Segments of DNA where the bases have been chemically modified by methylation may undergo a larger change in conformation and adopt the Z form. Here, the strands turn about the helical axis in a left-handed spiral, the opposite of the more common B form.[38] These unusual structures can be recognized by specific Z-DNA binding proteins and may be involved in the regulation of transcription.[39]

Quadruplex structures
[edit]At the ends of the linear chromosomes are specialized regions of DNA called telomeres. The main function of these regions is to allow the cell to replicate chromosome ends using the enzyme telomerase, as the enzymes that normally replicate DNA cannot copy the extreme 3′ ends of chromosomes.[41] These specialized chromosome caps also help protect the DNA ends, and stop the DNA repair systems in the cell from treating them as damage to be corrected.[42] In human cells, telomeres are usually lengths of single-stranded DNA containing several thousand repeats of a simple TTAGGG sequence.[43]
These guanine-rich sequences may stabilize chromosome ends by forming structures of stacked sets of four-base units, rather than the usual base pairs found in other DNA molecules. Here, four guanine bases form a flat plate and these flat four-base units then stack on top of each other, to form a stable G-quadruplex structure.[44] These structures are stabilized by hydrogen bonding between the edges of the bases and chelation of a metal ion in the centre of each four-base unit.[45] Other structures can also be formed, with the central set of four bases coming from either a single strand folded around the bases, or several different parallel strands, each contributing one base to the central structure.
In addition to these stacked structures, telomeres also form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle stabilized by telomere-binding proteins.[46] At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA and base pairing to one of the two strands. This triple-stranded structure is called a displacement loop or D-loop.[44]
Branched DNA
[edit]In DNA fraying occurs when non-complementary regions exist at the end of an otherwise complementary double-strand of DNA. However, branched DNA can occur if a third strand of DNA is introduced and contains adjoining regions able to hybridize with the frayed regions of the pre-existing double-strand. Although the simplest example of branched DNA involves only three strands of DNA, complexes involving additional strands and multiple branches are also possible.[47]
-
A DNA structure with a single branching point.
-
A DNA structure with multiple branches.
Chemical modifications
[edit]
|

|
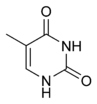
|
| cytosine | 5-methylcytosine | thymine |
Base modifications
[edit]The expression of genes is influenced by how the DNA is packaged in chromosomes, in a structure called chromatin. Base modifications can be involved in packaging, with regions that have low or no gene expression usually containing high levels of methylation of cytosine bases. For example, cytosine methylation, produces 5-methylcytosine, which is important for X-chromosome inactivation.[48] The average level of methylation varies between organisms - the worm Caenorhabditis elegans lacks cytosine methylation, while vertebrates have higher levels, with up to 1% of their DNA containing 5-methylcytosine.[49] Despite the importance of 5-methylcytosine, it can deaminate to leave a thymine base, methylated cytosines are therefore particularly prone to mutations.[50] Other base modifications include adenine methylation in bacteria, the presence of 5-hydroxymethylcytosine in the brain,[51] and the glycosylation of uracil to produce the "J-base" in kinetoplastids.[52][53]
Damage
[edit]
DNA can be damaged by many different sorts of mutagens, which change the DNA sequence. Mutagens include oxidizing agents, alkylating agents and also high-energy electromagnetic radiation such as ultraviolet light and X-rays. The type of DNA damage produced depends on the type of mutagen. For example, UV light can damage DNA by producing thymine dimers, which are cross-links between pyrimidine bases.[55] On the other hand, oxidants such as free radicals or hydrogen peroxide produce multiple forms of damage, including base modifications, particularly of guanosine, and double-strand breaks.[56] A typical human cell contains about 150,000 bases that have suffered oxidative damage.[57] Of these oxidative lesions, the most dangerous are double-strand breaks, as these are difficult to repair and can produce point mutations, insertions and deletions from the DNA sequence, as well as chromosomal translocations.[58]
Many mutagens fit into the space between two adjacent base pairs, this is called intercalating. Most intercalators are aromatic and planar molecules, and include Ethidium bromide, daunomycin, and doxorubicin. In order for an intercalator to fit between base pairs, the bases must separate, distorting the DNA strands by unwinding of the double helix. This inhibits both transcription and DNA replication, causing toxicity and mutations. As a result, DNA intercalators are often carcinogens, and Benzo[a]pyrene diol epoxide, acridines, aflatoxin and ethidium bromide are well-known examples.[59][60][61] Nevertheless, due to their ability to inhibit DNA transcription and replication, other similar toxins are also used in chemotherapy to inhibit rapidly growing cancer cells.[62]
Biological functions
[edit]DNA usually occurs as linear chromosomes in eukaryotes, and circular chromosomes in prokaryotes. The set of chromosomes in a cell makes up its genome; the human genome has approximately 3 billion base pairs of DNA arranged into 46 chromosomes.[63] The information carried by DNA is held in the sequence of pieces of DNA called genes. Transmission of genetic information in genes is achieved via complementary base pairing. For example, in transcription, when a cell uses the information in a gene, the DNA sequence is copied into a complementary RNA sequence through the attraction between the DNA and the correct RNA nucleotides. Usually, this RNA copy is then used to make a matching protein sequence in a process called translation which depends on the same interaction between RNA nucleotides. Alternatively, a cell may simply copy its genetic information in a process called DNA replication. The details of these functions are covered in other articles; here we focus on the interactions between DNA and other molecules that mediate the function of the genome.
Genes and genomes
[edit]Genomic DNA is located in the cell nucleus of eukaryotes, as well as small amounts in mitochondria and chloroplasts. In prokaryotes, the DNA is held within an irregularly shaped body in the cytoplasm called the nucleoid.[64] The genetic information in a genome is held within genes, and the complete set of this information in an organism is called its genotype. A gene is a unit of heredity and is a region of DNA that influences a particular characteristic in an organism. Genes contain an open reading frame that can be transcribed, as well as regulatory sequences such as promoters and enhancers, which control the transcription of the open reading frame.
In many species, only a small fraction of the total sequence of the genome encodes protein. For example, only about 1.5% of the human genome consists of protein-coding exons, with over 50% of human DNA consisting of non-coding repetitive sequences.[65] The reasons for the presence of so much non-coding DNA in eukaryotic genomes and the extraordinary differences in genome size, or C-value, among species represent a long-standing puzzle known as the "C-value enigma."[66] However, DNA sequences that do not code protein may still encode functional non-coding RNA molecules, which are involved in the regulation of gene expression.[67]

Some non-coding DNA sequences play structural roles in chromosomes. Telomeres and centromeres typically contain few genes, but are important for the function and stability of chromosomes.[42][69] An abundant form of non-coding DNA in humans are pseudogenes, which are copies of genes that have been disabled by mutation.[70] These sequences are usually just molecular fossils, although they can occasionally serve as raw genetic material for the creation of new genes through the process of gene duplication and divergence.[71]
Transcription and translation
[edit]A gene is a sequence of DNA that contains genetic information and can influence the phenotype of an organism. Within a gene, the sequence of bases along a DNA strand defines a messenger RNA sequence, which then defines one or more protein sequences. The relationship between the nucleotide sequences of genes and the amino-acid sequences of proteins is determined by the rules of translation, known collectively as the genetic code. The genetic code consists of three-letter 'words' called codons formed from a sequence of three nucleotides (e.g. ACT, CAG, TTT).
In transcription, the codons of a gene are copied into messenger RNA by RNA polymerase. This RNA copy is then decoded by a ribosome that reads the RNA sequence by base-pairing the messenger RNA to transfer RNA, which carries amino acids. Since there are 4 bases in 3-letter combinations, there are 64 possible codons ( combinations). These encode the twenty standard amino acids, giving most amino acids more than one possible codon. There are also three 'stop' or 'nonsense' codons signifying the end of the coding region; these are the TAA, TGA and TAG codons.

Replication
[edit]Cell division is essential for an organism to grow, but when a cell divides it must replicate the DNA in its genome so that the two daughter cells have the same genetic information as their parent. The double-stranded structure of DNA provides a simple mechanism for DNA replication. Here, the two strands are separated and then each strand's complementary DNA sequence is recreated by an enzyme called DNA polymerase. This enzyme makes the complementary strand by finding the correct base through complementary base pairing, and bonding it onto the original strand. As DNA polymerases can only extend a DNA strand in a 5′ to 3′ direction, different mechanisms are used to copy the antiparallel strands of the double helix.[72] In this way, the base on the old strand dictates which base appears on the new strand, and the cell ends up with a perfect copy of its DNA.
Interactions with proteins
[edit]All the functions of DNA depend on interactions with proteins. These protein interactions can be non-specific, or the protein can bind specifically to a single DNA sequence. Enzymes can also bind to DNA and of these, the polymerases that copy the DNA base sequence in transcription and DNA replication are particularly important.
DNA-binding proteins
[edit]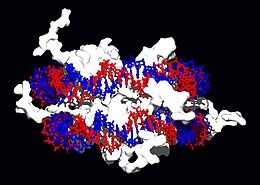
|
Structural proteins that bind DNA are well-understood examples of non-specific DNA-protein interactions. Within chromosomes, DNA is held in complexes with structural proteins. These proteins organize the DNA into a compact structure called chromatin. In eukaryotes this structure involves DNA binding to a complex of small basic proteins called histones, while in prokaryotes multiple types of proteins are involved.[73][74] The histones form a disk-shaped complex called a nucleosome, which contains two complete turns of double-stranded DNA wrapped around its surface. These non-specific interactions are formed through basic residues in the histones making ionic bonds to the acidic sugar-phosphate backbone of the DNA, and are therefore largely independent of the base sequence.[75] Chemical modifications of these basic amino acid residues include methylation, phosphorylation and acetylation.[76] These chemical changes alter the strength of the interaction between the DNA and the histones, making the DNA more or less accessible to transcription factors and changing the rate of transcription.[77] Other non-specific DNA-binding proteins in chromatin include the high-mobility group proteins, which bind to bent or distorted DNA.[78] These proteins are important in bending arrays of nucleosomes and arranging them into the larger structures that make up chromosomes.[79]
A distinct group of DNA-binding proteins are the DNA-binding proteins that specifically bind single-stranded DNA. In humans, replication protein A is the best-understood member of this family and is used in processes where the double helix is separated, including DNA replication, recombination and DNA repair.[80] These binding proteins seem to stabilize single-stranded DNA and protect it from forming stem-loops or being degraded by nucleases.

In contrast, other proteins have evolved to bind to particular DNA sequences. The most intensively studied of these are the various transcription factors, which are proteins that regulate transcription. Each transcription factor binds to one particular set of DNA sequences and activates or inhibits the transcription of genes that have these sequences close to their promoters. The transcription factors do this in two ways. Firstly, they can bind the RNA polymerase responsible for transcription, either directly or through other mediator proteins; this locates the polymerase at the promoter and allows it to begin transcription.[82] Alternatively, transcription factors can bind enzymes that modify the histones at the promoter; this will change the accessibility of the DNA template to the polymerase.[83]
As these DNA targets can occur throughout an organism's genome, changes in the activity of one type of transcription factor can affect thousands of genes.[84] Consequently, these proteins are often the targets of the signal transduction processes that control responses to environmental changes or cellular differentiation and development. The specificity of these transcription factors' interactions with DNA come from the proteins making multiple contacts to the edges of the DNA bases, allowing them to "read" the DNA sequence. Most of these base-interactions are made in the major groove, where the bases are most accessible.[85]

DNA-modifying enzymes
[edit]Nucleases and ligases
[edit]Nucleases are enzymes that cut DNA strands by catalyzing the hydrolysis of the phosphodiester bonds. Nucleases that hydrolyse nucleotides from the ends of DNA strands are called exonucleases, while endonucleases cut within strands. The most frequently used nucleases in molecular biology are the restriction endonucleases, which cut DNA at specific sequences. For instance, the EcoRV enzyme shown to the left recognizes the 6-base sequence 5′-GAT|ATC-3′ and makes a cut at the vertical line. In nature, these enzymes protect bacteria against phage infection by digesting the phage DNA when it enters the bacterial cell, acting as part of the restriction modification system.[87] In technology, these sequence-specific nucleases are used in molecular cloning and DNA fingerprinting.
Enzymes called DNA ligases can rejoin cut or broken DNA strands.[88] Ligases are particularly important in lagging strand DNA replication, as they join together the short segments of DNA produced at the replication fork into a complete copy of the DNA template. They are also used in DNA repair and genetic recombination.[88]
Topoisomerases and helicases
[edit]Topoisomerases are enzymes with both nuclease and ligase activity. These proteins change the amount of supercoiling in DNA. Some of these enzyme work by cutting the DNA helix and allowing one section to rotate, thereby reducing its level of supercoiling; the enzyme then seals the DNA break.[25] Other types of these enzymes are capable of cutting one DNA helix and then passing a second strand of DNA through this break, before rejoining the helix.[89] Topoisomerases are required for many processes involving DNA, such as DNA replication and transcription.[26]
Helicases are proteins that are a type of molecular motor. They use the chemical energy in nucleoside triphosphates, predominantly ATP, to break hydrogen bonds between bases and unwind the DNA double helix into single strands.[90] These enzymes are essential for most processes where enzymes need to access the DNA bases.
Polymerases
[edit]Polymerases are enzymes that synthesize polynucleotide chains from nucleoside triphosphates. The sequence of their products are copies of existing polynucleotide chains - which are called templates. These enzymes function by adding nucleotides onto the 3′ hydroxyl group of the previous nucleotide in a DNA strand. Consequently, all polymerases work in a 5′ to 3′ direction.[91] In the active site of these enzymes, the incoming nucleoside triphosphate base-pairs to the template: this allows polymerases to accurately synthesize the complementary strand of their template. Polymerases are classified according to the type of template that they use.
In DNA replication, a DNA-dependent DNA polymerase makes a copy of a DNA sequence. Accuracy is vital in this process, so many of these polymerases have a proofreading activity. Here, the polymerase recognizes the occasional mistakes in the synthesis reaction by the lack of base pairing between the mismatched nucleotides. If a mismatch is detected, a 3′ to 5′ exonuclease activity is activated and the incorrect base removed.[92] In most organisms DNA polymerases function in a large complex called the replisome that contains multiple accessory subunits, such as the DNA clamp or helicases.[93]
RNA-dependent DNA polymerases are a specialized class of polymerases that copy the sequence of an RNA strand into DNA. They include reverse transcriptase, which is a viral enzyme involved in the infection of cells by retroviruses, and telomerase, which is required for the replication of telomeres.[41][94] Telomerase is an unusual polymerase because it contains its own RNA template as part of its structure.[42]
Transcription is carried out by a DNA-dependent RNA polymerase that copies the sequence of a DNA strand into RNA. To begin transcribing a gene, the RNA polymerase binds to a sequence of DNA called a promoter and separates the DNA strands. It then copies the gene sequence into a messenger RNA transcript until it reaches a region of DNA called the terminator, where it halts and detaches from the DNA. As with human DNA-dependent DNA polymerases, RNA polymerase II, the enzyme that transcribes most of the genes in the human genome, operates as part of a large protein complex with multiple regulatory and accessory subunits.[95]
Genetic recombination
[edit]
|

|
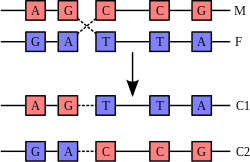
A DNA helix usually does not interact with other segments of DNA, and in human cells the different chromosomes even occupy separate areas in the nucleus called "chromosome territories".[97] This physical separation of different chromosomes is important for the ability of DNA to function as a stable repository for information, as one of the few times chromosomes interact is during chromosomal crossover when they recombine. Chromosomal crossover is when two DNA helices break, swap a section and then rejoin.
Recombination allows chromosomes to exchange genetic information and produces new combinations of genes, which increases the efficiency of natural selection and can be important in the rapid evolution of new proteins.[98] Genetic recombination can also be involved in DNA repair, particularly in the cell's response to double-strand breaks.[99]
The most common form of chromosomal crossover is homologous recombination, where the two chromosomes involved share very similar sequences. Non-homologous recombination can be damaging to cells, as it can produce chromosomal translocations and genetic abnormalities. The recombination reaction is catalyzed by enzymes known as recombinases, such as RAD51.[100] The first step in recombination is a double-stranded break either caused by an endonuclease or damage to the DNA.[101] A series of steps catalyzed in part by the recombinase then leads to joining of the two helices by at least one Holliday junction, in which a segment of a single strand in each helix is annealed to the complementary strand in the other helix. The Holliday junction is a tetrahedral junction structure that can be moved along the pair of chromosomes, swapping one strand for another. The recombination reaction is then halted by cleavage of the junction and re-ligation of the released DNA.[102]
Evolution
[edit]DNA contains the genetic information that allows all modern living things to function, grow and reproduce. However, it is unclear how long in the 4-billion-year history of life DNA has performed this function, as it has been proposed that the earliest forms of life may have used RNA as their genetic material.[91][103] RNA may have acted as the central part of early cell metabolism as it can both transmit genetic information and carry out catalysis as part of ribozymes.[104] This ancient RNA world where nucleic acid would have been used for both catalysis and genetics may have influenced the evolution of the current genetic code based on four nucleotide bases. This would occur since the number of unique bases in such an organism is a trade-off between a small number of bases increasing replication accuracy and a large number of bases increasing the catalytic efficiency of ribozymes.[105]
Unfortunately, there is no direct evidence of ancient genetic systems, as recovery of DNA from most fossils is impossible. This is because DNA will survive in the environment for less than one million years and slowly degrades into short fragments in solution.[106] Claims for older DNA have been made, most notably a report of the isolation of a viable bacterium from a salt crystal 250-million years old,[107] but these claims are controversial.[108][109]
Uses in technology
[edit]Genetic engineering
[edit]Methods have been developed to purify DNA from organisms, such as phenol-chloroform extraction and manipulate it in the laboratory, such as restriction digests and the polymerase chain reaction. Modern biology and biochemistry make intensive use of these techniques in recombinant DNA technology. Recombinant DNA is a man-made DNA sequence that has been assembled from other DNA sequences. They can be transformed into organisms in the form of plasmids or in the appropriate format, by using a viral vector.[110] The genetically modified organisms produced can be used to produce products such as recombinant proteins, used in medical research,[111] or be grown in agriculture.[112][113]
Forensics
[edit]Forensic scientists can use DNA in blood, semen, skin, saliva or hair found at a crime scene to identify a matching DNA of an individual, such as a perpetrator. This process is called genetic fingerprinting, or more accurately, DNA profiling. In DNA profiling, the lengths of variable sections of repetitive DNA, such as short tandem repeats and minisatellites, are compared between people. This method is usually an extremely reliable technique for identifying a matching DNA.[114] However, identification can be complicated if the scene is contaminated with DNA from several people.[115] DNA profiling was developed in 1984 by British geneticist Sir Alec Jeffreys,[116] and first used in forensic science to convict Colin Pitchfork in the 1988 Enderby murders case.[117]
People convicted of certain types of crimes may be required to provide a sample of DNA for a database. This has helped investigators solve old cases where only a DNA sample was obtained from the scene. DNA profiling can also be used to identify victims of mass casualty incidents.[118] On the other hand, many convicted people have been released from prison on the basis of DNA techniques, which were not available when a crime had originally been committed.
Bioinformatics
[edit]Bioinformatics involves the manipulation, searching, and data mining of DNA sequence data. The development of techniques to store and search DNA sequences have led to widely applied advances in computer science, especially string searching algorithms, machine learning and database theory.[119] String searching or matching algorithms, which find an occurrence of a sequence of letters inside a larger sequence of letters, were developed to search for specific sequences of nucleotides.[120] In other applications such as text editors, even simple algorithms for this problem usually suffice, but DNA sequences cause these algorithms to exhibit near-worst-case behaviour due to their small number of distinct characters. The related problem of sequence alignment aims to identify homologous sequences and locate the specific mutations that make them distinct. These techniques, especially multiple sequence alignment, are used in studying phylogenetic relationships and protein function.[121] Data sets representing entire genomes' worth of DNA sequences, such as those produced by the Human Genome Project, are difficult to use without annotations, which label the locations of genes and regulatory elements on each chromosome. Regions of DNA sequence that have the characteristic patterns associated with protein- or RNA-coding genes can be identified by gene finding algorithms, which allow researchers to predict the presence of particular gene products in an organism even before they have been isolated experimentally.[122]
DNA nanotechnology
[edit]
DNA nanotechnology uses the unique molecular recognition properties of DNA and other nucleic acids to create self-assembling branched DNA complexes with useful properties.[123] DNA is thus used as a structural material rather than as a carrier of biological information. This has led to the creation of two-dimensional periodic lattices (both tile-based as well as using the "DNA origami" method) as well as three-dimensional structures in the shapes of polyhedra.[124] Nanomechanical devices and algorithmic self-assembly have also been demonstrated,[125] and these DNA structures have been used to template the arrangement of other molecules such as gold nanoparticles and streptavidin proteins.[126]
History and anthropology
[edit]Because DNA collects mutations over time, which are then inherited, it contains historical information and by comparing DNA sequences, geneticists can infer the evolutionary history of organisms, their phylogeny.[127] This field of phylogenetics is a powerful tool in evolutionary biology. If DNA sequences within a species are compared, population geneticists can learn the history of particular populations. This can be used in studies ranging from ecological genetics to anthropology; for example, DNA evidence is being used to try to identify the Ten Lost Tribes of Israel.[128][129]
DNA has also been used to look at modern family relationships, such as establishing family relationships between the descendants of Sally Hemings and Thomas Jefferson. This usage is closely related to the use of DNA in criminal investigations detailed above. Indeed, some criminal investigations have been solved when DNA from crime scenes has matched relatives of the guilty individual.[130]
History of DNA research
[edit]DNA was first isolated by the Swiss physician Friedrich Miescher who, in 1869, discovered a microscopic substance in the pus of discarded surgical bandages. As it resided in the nuclei of cells, he called it "nuclein".[131] In 1919, Phoebus Levene identified the base, sugar and phosphate nucleotide unit.[132] Levene suggested that DNA consisted of a string of nucleotide units linked together through the phosphate groups. However, Levene thought the chain was short and the bases repeated in a fixed order. In 1937 William Astbury produced the first X-ray diffraction patterns that showed that DNA had a regular structure.[133]
In 1928, Frederick Griffith discovered that traits of the "smooth" form of the Pneumococcus could be transferred to the "rough" form of the same bacteria by mixing killed "smooth" bacteria with the live "rough" form.[134] This system provided the first clear suggestion that DNA carried genetic information—the Avery-MacLeod-McCarty experiment—when Oswald Avery, along with coworkers Colin MacLeod and Maclyn McCarty, identified DNA as the transforming principle in 1943.[135] DNA's role in heredity was confirmed in 1952, when Alfred Hershey and Martha Chase in the Hershey-Chase experiment showed that DNA is the genetic material of the T2 phage.[136]
-
DNA Helix controversy
In 1953 James D. Watson and Francis Crick suggested what is now accepted as the first correct double-helix model of DNA structure in the journal Nature.[6] Their double-helix, molecular model of DNA was then based on a single X-ray diffraction image (labeled as "Photo 51")[137] taken by Rosalind Franklin and Raymond Gosling in May 1952, as well as the information that the DNA bases were paired—also obtained through private communications from Erwin Chargaff in the previous years. Chargaff's rules played a very important role in establishing double-helix configurations for B-DNA as well as A-DNA.
Experimental evidence supporting the Watson and Crick model were published in a series of five articles in the same issue of Nature.[138] Of these, Franklin and Gosling's paper was the first publication of their own X-ray diffraction data and original analysis method that partially supported the Watson and Crick model[30][139]; this issue also contained an article on DNA structure by Maurice Wilkins and two of his colleagues, whose analysis and in vivo B-DNA X-ray patterns also supported the presence in vivo of the double-helical DNA configurations as proposed by Crick and Watson for their double-helix molecular model of DNA in the previous two pages of Nature.[31] In 1962, after Franklin's death, Watson, Crick, and Wilkins jointly received the Nobel Prize in Physiology or Medicine.[140] Unfortunately, Nobel rules of the time allowed only living recipients, but a vigorous debate continues on who should receive credit for the discovery.[141]
In an influential presentation in 1957, Crick laid out the "Central Dogma" of molecular biology, which foretold the relationship between DNA, RNA, and proteins, and articulated the "adaptor hypothesis".[142] Final confirmation of the replication mechanism that was implied by the double-helical structure followed in 1958 through the Meselson-Stahl experiment.[143] Further work by Crick and coworkers showed that the genetic code was based on non-overlapping triplets of bases, called codons, allowing Har Gobind Khorana, Robert W. Holley and Marshall Warren Nirenberg to decipher the genetic code.[144] These findings represent the birth of molecular biology.
See also
[edit]Notes
[edit]- ^ Saenger, Wolfram (1984). Principles of Nucleic Acid Structure. New York: Springer-Verlag. ISBN 0387907629.
- ^ a b Alberts, Bruce (2002). Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 0-8153-3218-1. OCLC 145080076 48122761 57023651 69932405.
{{cite book}}: Check|oclc=value (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Butler, John M. (2001). Forensic DNA Typing. Elsevier. ISBN 978-0-12-147951-0. OCLC 223032110 45406517.
{{cite book}}: Check|oclc=value (help) pp. 14–15. - ^ Mandelkern M, Elias J, Eden D, Crothers D (1981). "The dimensions of DNA in solution". J Mol Biol. 152 (1): 153–61. doi:10.1016/0022-2836(81)90099-1. PMID 7338906.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gregory S; et al. (2006). "The DNA sequence and biological annotation of human chromosome 1". Nature. 441 (7091): 315–21. doi:10.1038/nature04727. PMID 16710414. S2CID 4431933.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b c Watson J.D. and Crick F.H.C. (1953). "A Structure for Deoxyribose Nucleic Acid" (PDF). Nature. 171 (4356): 737–738. doi:10.1038/171737a0. PMID 13054692. S2CID 4253007. Retrieved 4 May 2009.
- ^ a b c Berg J., Tymoczko J. and Stryer L. (2002) Biochemistry. W. H. Freeman and Company ISBN 0-7167-4955-6
- ^ Abbreviations and Symbols for Nucleic Acids, Polynucleotides and their Constituents IUPAC-IUB Commission on Biochemical Nomenclature (CBN), Accessed 03 January 2006
- ^ a b Ghosh A, Bansal M (2003). "A glossary of DNA structures from A to Z". Acta Crystallogr D Biol Crystallogr. 59 (Pt 4): 620–6. doi:10.1107/S0907444903003251. PMID 12657780.
- ^ Created from PDB 1D65
- ^ Wing R, Drew H, Takano T, Broka C, Tanaka S, Itakura K, Dickerson R (1980). "Crystal structure analysis of a complete turn of B-DNA". Nature. 287 (5784): 755–8. doi:10.1038/287755a0. PMID 7432492. S2CID 4315465.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pabo C, Sauer R (1984). "Protein-DNA recognition". Annu Rev Biochem. 53: 293–321. doi:10.1146/annurev.bi.53.070184.001453. PMID 6236744.
- ^ Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub H (2000). "Mechanical stability of single DNA molecules". Biophys J. 78 (4): 1997–2007. doi:10.1016/S0006-3495(00)76747-6. PMC 1300792. PMID 10733978.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yakovchuk P, Protozanova E, Frank-Kamenetskii MD (2006). "Base-stacking and base-pairing contributions into thermal stability of the DNA double helix". Nucleic Acids Res. 34 (2): 564–74. doi:10.1093/nar/gkj454. PMC 1360284. PMID 16449200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chalikian T, Völker J, Plum G, Breslauer K (1999). "A more unified picture for the thermodynamics of nucleic acid duplex melting: a characterization by calorimetric and volumetric techniques". Proc Natl Acad Sci USA. 96 (14): 7853–8. doi:10.1073/pnas.96.14.7853. PMC 22151. PMID 10393911.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ deHaseth P, Helmann J (1995). "Open complex formation by Escherichia coli RNA polymerase: the mechanism of polymerase-induced strand separation of double helical DNA". Mol Microbiol. 16 (5): 817–24. doi:10.1111/j.1365-2958.1995.tb02309.x. PMID 7476180. S2CID 24479358.
- ^ Isaksson J, Acharya S, Barman J, Cheruku P, Chattopadhyaya J (2004). "Single-stranded adenine-rich DNA and RNA retain structural characteristics of their respective double-stranded conformations and show directional differences in stacking pattern". Biochemistry. 43 (51): 15996–6010. doi:10.1021/bi048221v. PMID 15609994.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Designation of the two strands of DNA JCBN/NC-IUB Newsletter 1989, Accessed 07 May 2008
- ^ Hüttenhofer A, Schattner P, Polacek N (2005). "Non-coding RNAs: hope or hype?". Trends Genet. 21 (5): 289–97. doi:10.1016/j.tig.2005.03.007. PMID 15851066.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Munroe S (2004). "Diversity of antisense regulation in eukaryotes: multiple mechanisms, emerging patterns". J Cell Biochem. 93 (4): 664–71. doi:10.1002/jcb.20252. PMID 15389973. S2CID 23748148.
- ^ Makalowska I, Lin C, Makalowski W (2005). "Overlapping genes in vertebrate genomes". Comput Biol Chem. 29 (1): 1–12. doi:10.1016/j.compbiolchem.2004.12.006. PMID 15680581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Johnson Z, Chisholm S (2004). "Properties of overlapping genes are conserved across microbial genomes". Genome Res. 14 (11): 2268–72. doi:10.1101/gr.2433104. PMC 525685. PMID 15520290.
- ^ Lamb R, Horvath C (1991). "Diversity of coding strategies in influenza viruses". Trends Genet. 7 (8): 261–6. doi:10.1016/0168-9525(91)90326-L. PMC 7173306. PMID 1771674.
- ^ Benham C, Mielke S (2005). "DNA mechanics". Annu Rev Biomed Eng. 7: 21–53. doi:10.1146/annurev.bioeng.6.062403.132016. PMID 16004565.
- ^ a b Champoux J (2001). "DNA topoisomerases: structure, function, and mechanism". Annu Rev Biochem. 70: 369–413. doi:10.1146/annurev.biochem.70.1.369. PMID 11395412.
- ^ a b Wang J (2002). "Cellular roles of DNA topoisomerases: a molecular perspective". Nat Rev Mol Cell Biol. 3 (6): 430–40. doi:10.1038/nrm831. PMID 12042765. S2CID 205496065.
- ^ X-ray images are courtesy of Dr. H. R. Wilson, F.R.S.Created from http://commons.wikimedia.org/wiki/File:ABDNAxrgpj.jpg
- ^ Basu H, Feuerstein B, Zarling D, Shafer R, Marton L (1988). "Recognition of Z-RNA and Z-DNA determinants by polyamines in solution: experimental and theoretical studies". J Biomol Struct Dyn. 6 (2): 299–309. doi:10.1080/07391102.1988.10507714. PMID 2482766.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Franklin RE, Gosling RG (6 March 1953). "The Structure of Sodium Thymonucleate Fibres I. The Influence of Water Content" (PDF). Acta Cryst. 6 (8–9): 673–7. doi:10.1107/S0365110X53001939.
Franklin RE, Gosling RG (September 1953). "The structure of sodium thymonucleate fibres. II. The cylindrically symmetrical Patterson function". Acta Cryst. 6 (8–9): 678–85. doi:10.1107/S0365110X53001940.{{cite journal}}: CS1 maint: date and year (link) - ^ a b Franklin, Rosalind and Gosling, Raymond (1953). "Molecular Configuration in Sodium Thymonucleate. Franklin R. and Gosling R.G" (PDF). Nature. 171 (4356): 740–1. doi:10.1038/171740a0. PMID 13054694. S2CID 4268222.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wilkins M.H.F., A.R. Stokes A.R. & Wilson, H.R. (1953). "Molecular Structure of Deoxypentose Nucleic Acids" (PDF). Nature. 171 (4356): 738–740. doi:10.1038/171738a0. PMID 13054693. S2CID 4280080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leslie AG, Arnott S, Chandrasekaran R, Ratliff RL (1980). "Polymorphism of DNA double helices". J. Mol. Biol. 143 (1): 49–72. doi:10.1016/0022-2836(80)90124-2. PMID 7441761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baianu, I.C. (1980). "Structural Order and Partial Disorder in Biological systems". Bull. Math. Biol. 42 (4): 137–141. doi:10.1016/S0092-8240(80)80083-8. S2CID 98166726. http://cogprints.org/3822/
- ^ Hosemann R., Bagchi R.N., Direct analysis of diffraction by matter, North-Holland Publs., Amsterdam – New York, 1962.
- ^ Baianu, I.C. (1978). "X-ray scattering by partially disordered membrane systems". Acta Cryst. A34 (5): 751–753. doi:10.1107/S0567739478001540.
- ^ Wahl M, Sundaralingam M (1997). "Crystal structures of A-DNA duplexes". Biopolymers. 44 (1): 45–63. doi:10.1002/(SICI)1097-0282(1997)44:1<45::AID-BIP4>3.0.CO;2-#. PMID 9097733.
- ^ Lu XJ, Shakked Z, Olson WK (2000). "A-form conformational motifs in ligand-bound DNA structures". J. Mol. Biol. 300 (4): 819–40. doi:10.1006/jmbi.2000.3690. PMID 10891271.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rothenburg S, Koch-Nolte F, Haag F (2001). "DNA methylation and Z-DNA formation as mediators of quantitative differences in the expression of alleles". Immunol Rev. 184: 286–98. doi:10.1034/j.1600-065x.2001.1840125.x. PMID 12086319. S2CID 20589136.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Oh D, Kim Y, Rich A (2002). "Z-DNA-binding proteins can act as potent effectors of gene expression in vivo". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16666–71. doi:10.1073/pnas.262672699. PMC 139201. PMID 12486233.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Created from NDB UD0017
- ^ a b Greider C, Blackburn E (1985). "Identification of a specific telomere terminal transferase activity in Tetrahymena extracts". Cell. 43 (2 Pt 1): 405–13. doi:10.1016/0092-8674(85)90170-9. PMID 3907856.
- ^ a b c Nugent C, Lundblad V (1998). "The telomerase reverse transcriptase: components and regulation". Genes Dev. 12 (8): 1073–85. doi:10.1101/gad.12.8.1073. PMID 9553037.
- ^ Wright W, Tesmer V, Huffman K, Levene S, Shay J (1997). "Normal human chromosomes have long G-rich telomeric overhangs at one end". Genes Dev. 11 (21): 2801–9. doi:10.1101/gad.11.21.2801. PMC 316649. PMID 9353250.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Burge S, Parkinson G, Hazel P, Todd A, Neidle S (2006). "Quadruplex DNA: sequence, topology and structure". Nucleic Acids Res. 34 (19): 5402–15. doi:10.1093/nar/gkl655. PMC 1636468. PMID 17012276.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Parkinson G, Lee M, Neidle S (2002). "Crystal structure of parallel quadruplexes from human telomeric DNA". Nature. 417 (6891): 876–80. doi:10.1038/nature755. PMID 12050675. S2CID 4422211.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Griffith J, Comeau L, Rosenfield S, Stansel R, Bianchi A, Moss H, de Lange T (1999). "Mammalian telomeres end in a large duplex loop". Cell. 97 (4): 503–14. doi:10.1016/S0092-8674(00)80760-6. PMID 10338214. S2CID 721901.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Seeman NC (November 2005). "DNA enables nanoscale control of the structure of matter". Q. Rev. Biophys. 38 (4): 363–71. doi:10.1017/S0033583505004087. PMC 3478329. PMID 16515737.
{{cite journal}}: CS1 maint: date and year (link) - ^ Klose R, Bird A (2006). "Genomic DNA methylation: the mark and its mediators". Trends Biochem Sci. 31 (2): 89–97. doi:10.1016/j.tibs.2005.12.008. PMID 16403636.
- ^ Bird A (2002). "DNA methylation patterns and epigenetic memory". Genes Dev. 16 (1): 6–21. doi:10.1101/gad.947102. PMID 11782440.
- ^ Walsh C, Xu G (2006). "Cytosine methylation and DNA repair". Curr Top Microbiol Immunol. Current Topics in Microbiology and Immunology. 301: 283–315. doi:10.1007/3-540-31390-7_11. ISBN 3-540-29114-8. PMID 16570853.
- ^ Kriaucionis S, Heintz N (May 2009). "The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain". Science. 324 (5929): 929–30. doi:10.1126/science.1169786. PMC 3263819. PMID 19372393.
{{cite journal}}: CS1 maint: date and year (link) - ^ Ratel D, Ravanat J, Berger F, Wion D (2006). "N6-methyladenine: the other methylated base of DNA". BioEssays. 28 (3): 309–15. doi:10.1002/bies.20342. PMC 2754416. PMID 16479578.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gommers-Ampt J, Van Leeuwen F, de Beer A, Vliegenthart J, Dizdaroglu M, Kowalak J, Crain P, Borst P (1993). "beta-D-glucosyl-hydroxymethyluracil: a novel modified base present in the DNA of the parasitic protozoan T. brucei". Cell. 75 (6): 1129–36. doi:10.1016/0092-8674(93)90322-H. hdl:1874/5219. PMID 8261512. S2CID 24801094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Created from PDB 1JDG
- ^ Douki T, Reynaud-Angelin A, Cadet J, Sage E (2003). "Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation". Biochemistry. 42 (30): 9221–6. doi:10.1021/bi034593c. PMID 12885257.
{{cite journal}}: CS1 maint: multiple names: authors list (link), - ^ Cadet J, Delatour T, Douki T, Gasparutto D, Pouget J, Ravanat J, Sauvaigo S (1999). "Hydroxyl radicals and DNA base damage". Mutat Res. 424 (1–2): 9–21. doi:10.1016/s0027-5107(99)00004-4. PMID 10064846.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Beckman KB, Ames BN (August 1997). "Oxidative decay of DNA". J. Biol. Chem. 272 (32): 19633–6. doi:10.1074/jbc.272.32.19633. PMID 9289489.
{{cite journal}}: CS1 maint: date and year (link) - ^ Valerie K, Povirk L (2003). "Regulation and mechanisms of mammalian double-strand break repair". Oncogene. 22 (37): 5792–812. doi:10.1038/sj.onc.1206679. PMID 12947387. S2CID 25299836.
- ^ Ferguson L, Denny W (1991). "The genetic toxicology of acridines". Mutat Res. 258 (2): 123–60. doi:10.1016/0165-1110(91)90006-h. PMID 1881402.
- ^ Jeffrey A (1985). "DNA modification by chemical carcinogens". Pharmacol Ther. 28 (2): 237–72. doi:10.1016/0163-7258(85)90013-0. PMID 3936066.
- ^ Stephens T, Bunde C, Fillmore B (2000). "Mechanism of action in thalidomide teratogenesis". Biochem Pharmacol. 59 (12): 1489–99. doi:10.1016/S0006-2952(99)00388-3. PMID 10799645.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Braña M, Cacho M, Gradillas A, de Pascual-Teresa B, Ramos A (2001). "Intercalators as anticancer drugs". Curr Pharm Des. 7 (17): 1745–80. doi:10.2174/1381612013397113. PMID 11562309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Venter J; et al. (2001). "The sequence of the human genome". Science. 291 (5507): 1304–51. doi:10.1126/science.1058040. PMID 11181995.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Thanbichler M, Wang S, Shapiro L (2005). "The bacterial nucleoid: a highly organized and dynamic structure". J Cell Biochem. 96 (3): 506–21. doi:10.1002/jcb.20519. PMID 15988757. S2CID 25355087.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolfsberg T, McEntyre J, Schuler G (2001). "Guide to the draft human genome". Nature. 409 (6822): 824–6. doi:10.1038/35057000. PMID 11236998. S2CID 4424567.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gregory T (2005). "The C-value enigma in plants and animals: a review of parallels and an appeal for partnership". Ann Bot (Lond). 95 (1): 133–46. doi:10.1093/aob/mci009. PMC 4246714. PMID 15596463.
- ^ The ENCODE Project Consortium (2007). "Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project". Nature. 447 (7146): 799–816. doi:10.1038/nature05874. PMC 2212820. PMID 17571346.
- ^ Created from PDB 1MSW
- ^ Pidoux A, Allshire R (2005). "The role of heterochromatin in centromere function". Philos Trans R Soc Lond B Biol Sci. 360 (1455): 569–79. doi:10.1098/rstb.2004.1611. PMC 1569473. PMID 15905142.
- ^ Harrison P, Hegyi H, Balasubramanian S, Luscombe N, Bertone P, Echols N, Johnson T, Gerstein M (2002). "Molecular fossils in the human genome: identification and analysis of the pseudogenes in chromosomes 21 and 22". Genome Res. 12 (2): 272–80. doi:10.1101/gr.207102. PMC 155275. PMID 11827946.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Harrison P, Gerstein M (2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution". J Mol Biol. 318 (5): 1155–74. doi:10.1016/S0022-2836(02)00109-2. PMID 12083509.
- ^ Albà M (2001). "Replicative DNA polymerases". Genome Biol. 2 (1): REVIEWS3002. doi:10.1186/gb-2001-2-1-reviews3002. PMC 150442. PMID 11178285.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sandman K, Pereira S, Reeve J (1998). "Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome". Cell Mol Life Sci. 54 (12): 1350–64. doi:10.1007/s000180050259. PMID 9893710. S2CID 21101836.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dame RT (2005). "The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin". Mol. Microbiol. 56 (4): 858–70. doi:10.1111/j.1365-2958.2005.04598.x. PMID 15853876. S2CID 26965112.
- ^ Luger K, Mäder A, Richmond R, Sargent D, Richmond T (1997). "Crystal structure of the nucleosome core particle at 2.8 A resolution". Nature. 389 (6648): 251–60. doi:10.1038/38444. PMID 9305837. S2CID 4328827.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jenuwein T, Allis C (2001). "Translating the histone code". Science. 293 (5532): 1074–80. doi:10.1126/science.1063127. PMID 11498575. S2CID 1883924.
- ^ Ito T (2003). "Nucleosome assembly and remodelling". Curr Top Microbiol Immunol. Current Topics in Microbiology and Immunology. 274: 1–22. doi:10.1007/978-3-642-55747-7_1. ISBN 978-3-642-62909-9. PMID 12596902.
- ^ Thomas J (2001). "HMG1 and 2: architectural DNA-binding proteins". Biochem Soc Trans. 29 (Pt 4): 395–401. doi:10.1042/BST0290395. PMID 11497996.
- ^ Grosschedl R, Giese K, Pagel J (1994). "HMG domain proteins: architectural elements in the assembly of nucleoprotein structures". Trends Genet. 10 (3): 94–100. doi:10.1016/0168-9525(94)90232-1. PMID 8178371.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Iftode C, Daniely Y, Borowiec J (1999). "Replication protein A (RPA): the eukaryotic SSB". Crit Rev Biochem Mol Biol. 34 (3): 141–80. doi:10.1080/10409239991209255. PMID 10473346.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Created from PDB 1LMB
- ^ Myers L, Kornberg R (2000). "Mediator of transcriptional regulation". Annu Rev Biochem. 69: 729–49. doi:10.1146/annurev.biochem.69.1.729. PMID 10966474.
- ^ Spiegelman B, Heinrich R (2004). "Biological control through regulated transcriptional coactivators". Cell. 119 (2): 157–67. doi:10.1016/j.cell.2004.09.037. PMID 15479634. S2CID 14668705.
- ^ Li Z, Van Calcar S, Qu C, Cavenee W, Zhang M, Ren B (2003). "A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells". Proc Natl Acad Sci USA. 100 (14): 8164–9. doi:10.1073/pnas.1332764100. PMC 166200. PMID 12808131.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pabo C, Sauer R (1984). "Protein-DNA recognition". Annu Rev Biochem. 53: 293–321. doi:10.1146/annurev.bi.53.070184.001453. PMID 6236744.
- ^ Created from PDB 1RVA
- ^ Bickle T, Krüger D (1993). "Biology of DNA restriction". Microbiol Rev. 57 (2): 434–50. doi:10.1128/mr.57.2.434-450.1993. PMC 372918. PMID 8336674.
- ^ a b Doherty A, Suh S (2000). "Structural and mechanistic conservation in DNA ligases". Nucleic Acids Res. 28 (21): 4051–8. doi:10.1093/nar/28.21.4051. PMC 113121. PMID 11058099.
- ^ Schoeffler A, Berger J (2005). "Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism". Biochem Soc Trans. 33 (Pt 6): 1465–70. doi:10.1042/BST20051465. PMID 16246147.
- ^ Tuteja N, Tuteja R (2004). "Unraveling DNA helicases. Motif, structure, mechanism and function". Eur J Biochem. 271 (10): 1849–63. doi:10.1111/j.1432-1033.2004.04094.x. PMID 15128295.
- ^ a b Joyce C, Steitz T (1995). "Polymerase structures and function: variations on a theme?". J Bacteriol. 177 (22): 6321–9. doi:10.1128/jb.177.22.6321-6329.1995. PMC 177480. PMID 7592405. Cite error: The named reference "Joyce" was defined multiple times with different content (see the help page).
- ^ Hubscher U, Maga G, Spadari S (2002). "Eukaryotic DNA polymerases". Annu Rev Biochem. 71: 133–63. doi:10.1146/annurev.biochem.71.090501.150041. PMID 12045093.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Johnson A, O'Donnell M (2005). "Cellular DNA replicases: components and dynamics at the replication fork". Annu Rev Biochem. 74: 283–315. doi:10.1146/annurev.biochem.73.011303.073859. PMID 15952889.
- ^ Tarrago-Litvak L, Andréola M, Nevinsky G, Sarih-Cottin L, Litvak S (1 May 1994). "The reverse transcriptase of HIV-1: from enzymology to therapeutic intervention". FASEB J. 8 (8): 497–503. doi:10.1096/fasebj.8.8.7514143. PMID 7514143. S2CID 39614573.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Martinez E (2002). "Multi-protein complexes in eukaryotic gene transcription". Plant Mol Biol. 50 (6): 925–47. doi:10.1023/A:1021258713850. PMID 12516863. S2CID 24946189.
- ^ Created from PDB 1M6G
- ^ Cremer T, Cremer C (2001). "Chromosome territories, nuclear architecture and gene regulation in mammalian cells". Nat Rev Genet. 2 (4): 292–301. doi:10.1038/35066075. PMID 11283701. S2CID 8547149.
- ^ Pál C, Papp B, Lercher M (2006). "An integrated view of protein evolution". Nat Rev Genet. 7 (5): 337–48. doi:10.1038/nrg1838. PMID 16619049. S2CID 23225873.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ O'Driscoll M, Jeggo P (2006). "The role of double-strand break repair - insights from human genetics". Nat Rev Genet. 7 (1): 45–54. doi:10.1038/nrg1746. PMID 16369571. S2CID 7779574.
- ^ Vispé S, Defais M (1997). "Mammalian Rad51 protein: a RecA homologue with pleiotropic functions". Biochimie. 79 (9–10): 587–92. doi:10.1016/S0300-9084(97)82007-X. PMID 9466696.
- ^ Neale MJ, Keeney S (2006). "Clarifying the mechanics of DNA strand exchange in meiotic recombination". Nature. 442 (7099): 153–8. doi:10.1038/nature04885. PMC 5607947. PMID 16838012.
- ^ Dickman M, Ingleston S, Sedelnikova S, Rafferty J, Lloyd R, Grasby J, Hornby D (2002). "The RuvABC resolvasome". Eur J Biochem. 269 (22): 5492–501. doi:10.1046/j.1432-1033.2002.03250.x. PMID 12423347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Orgel L (2004). "Prebiotic chemistry and the origin of the RNA world" (PDF). Crit Rev Biochem Mol Biol. 39 (2): 99–123. doi:10.1080/10409230490460765. PMID 15217990.
- ^ Davenport R (2001). "Ribozymes. Making copies in the RNA world". Science. 292 (5520): 1278. doi:10.1126/science.292.5520.1278a. PMID 11360970. S2CID 85976762.
- ^ Szathmáry E (1992). "What is the optimum size for the genetic alphabet?" (PDF). Proc Natl Acad Sci USA. 89 (7): 2614–8. doi:10.1073/pnas.89.7.2614. PMC 48712. PMID 1372984.
- ^ Lindahl T (1993). "Instability and decay of the primary structure of DNA". Nature. 362 (6422): 709–15. doi:10.1038/362709a0. PMID 8469282. S2CID 4283694.
- ^ Vreeland R, Rosenzweig W, Powers D (2000). "Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal". Nature. 407 (6806): 897–900. doi:10.1038/35038060. PMID 11057666. S2CID 9879073.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hebsgaard M, Phillips M, Willerslev E (2005). "Geologically ancient DNA: fact or artefact?". Trends Microbiol. 13 (5): 212–20. doi:10.1016/j.tim.2005.03.010. PMID 15866038.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nickle D, Learn G, Rain M, Mullins J, Mittler J (2002). "Curiously modern DNA for a "250 million-year-old" bacterium". J Mol Evol. 54 (1): 134–7. doi:10.1007/s00239-001-0025-x. PMID 11734907. S2CID 24740859.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goff SP, Berg P (1976). "Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells". Cell. 9 (4 PT 2): 695–705. doi:10.1016/0092-8674(76)90133-1. PMID 189942. S2CID 41788896.
- ^ Houdebine L (2007). "Transgenic animal models in biomedical research". Target Discovery and Validation Reviews and Protocols. Methods Mol Biol. Vol. 360. pp. 163–202. doi:10.1385/1-59745-165-7:163. ISBN 978-1-59745-165-9. PMID 17172731.
- ^ Daniell H, Dhingra A (2002). "Multigene engineering: dawn of an exciting new era in biotechnology". Curr Opin Biotechnol. 13 (2): 136–41. doi:10.1016/S0958-1669(02)00297-5. PMC 3481857. PMID 11950565.
- ^ Job D (2002). "Plant biotechnology in agriculture". Biochimie. 84 (11): 1105–10. doi:10.1016/S0300-9084(02)00013-5. PMID 12595138.
- ^ Collins A, Morton N (1994). "Likelihood ratios for DNA identification" (PDF). Proc Natl Acad Sci USA. 91 (13): 6007–11. doi:10.1073/pnas.91.13.6007. PMC 44126. PMID 8016106.
- ^ Weir B, Triggs C, Starling L, Stowell L, Walsh K, Buckleton J (1997). "Interpreting DNA mixtures". J Forensic Sci. 42 (2): 213–22. doi:10.1520/JFS14100J. PMID 9068179.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jeffreys A, Wilson V, Thein S (1985). "Individual-specific 'fingerprints' of human DNA". Nature. 316 (6023): 76–9. doi:10.1038/316076a0. PMID 2989708. S2CID 4229883.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Colin Pitchfork — first murder conviction on DNA evidence also clears the prime suspect Forensic Science Service Accessed 23 December 2006
- ^ "DNA Identification in Mass Fatality Incidents". National Institute of Justice. September 2006.
{{cite web}}: CS1 maint: date and year (link) - ^ Baldi, Pierre; Brunak, Soren (2001), Bioinformatics: The Machine Learning Approach, MIT Press, ISBN 978-0-262-02506-5, OCLC 45951728 57562233
{{citation}}: Check|oclc=value (help). - ^ Gusfield, Dan. Algorithms on Strings, Trees, and Sequences: Computer Science and Computational Biology. Cambridge University Press, 15 January 1997. ISBN 978-0-521-58519-4.
- ^ Sjölander K (2004). "Phylogenomic inference of protein molecular function: advances and challenges". Bioinformatics. 20 (2): 170–9. doi:10.1093/bioinformatics/bth021. PMID 14734307.
- ^ Mount DM (2004). Bioinformatics: Sequence and Genome Analysis (2 ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. ISBN 0879697121. OCLC 55106399.
- ^ Rothemund PW (March 2006). "Folding DNA to create nanoscale shapes and patterns" (PDF). Nature. 440 (7082): 297–302. doi:10.1038/nature04586. PMID 16541064. S2CID 4316391.
{{cite journal}}: CS1 maint: date and year (link) - ^ Andersen ES, Dong M, Nielsen MM; et al. (May 2009). "Self-assembly of a nanoscale DNA box with a controllable lid". Nature. 459 (7243): 73–6. doi:10.1038/nature07971. hdl:11858/00-001M-0000-0010-9363-9. PMID 19424153. S2CID 4430815.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Ishitsuka Y, Ha T (May 2009). "DNA nanotechnology: a nanomachine goes live". Nat Nanotechnol. 4 (5): 281–2. doi:10.1038/nnano.2009.101. PMID 19421208.
{{cite journal}}: CS1 maint: date and year (link) - ^ Aldaye FA, Palmer AL, Sleiman HF (September 2008). "Assembling materials with DNA as the guide". Science. 321 (5897): 1795–9. doi:10.1126/science.1154533. PMID 18818351. S2CID 2755388.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Wray G (2002). "Dating branches on the tree of life using DNA". Genome Biol. 3 (1): REVIEWS0001. doi:10.1046/j.1525-142X.1999.99010.x. PMC 150454. PMID 11806830.
- ^ Lost Tribes of Israel, NOVA, PBS airdate: 22 February 2000. Transcript available from PBS.org, (last accessed on 4 March 2006)
- ^ Kleiman, Yaakov. "The Cohanim/DNA Connection: The fascinating story of how DNA studies confirm an ancient biblical tradition". aish.com (January 13, 2000). Accessed 4 March 2006.
- ^ Bhattacharya, Shaoni. "Killer convicted thanks to relative's DNA". newscientist.com (20 April 2004). Accessed 22 December 06
- ^ Dahm R (January 2008). "Discovering DNA: Friedrich Miescher and the early years of nucleic acid research". Hum. Genet. 122 (6): 565–81. doi:10.1007/s00439-007-0433-0. PMID 17901982. S2CID 915930.
{{cite journal}}: CS1 maint: date and year (link) - ^ Levene P (1 December 1919). "The structure of yeast nucleic acid". J Biol Chem. 40 (2): 415–24. doi:10.1016/S0021-9258(18)87254-4.
- ^ Astbury W (1947). "Nucleic acid". Symp. SOC. Exp. BBL. 1 (66).
- ^ Lorenz MG, Wackernagel W (1 September 1994). "Bacterial gene transfer by natural genetic transformation in the environment". Microbiol. Rev. 58 (3): 563–602. doi:10.1128/mr.58.3.563-602.1994. PMC 372978. PMID 7968924.
- ^ Avery O, MacLeod C, McCarty M (1944). "Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Inductions of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III". J Exp Med. 79 (2): 137–158. doi:10.1084/jem.79.2.137. PMC 2135445. PMID 19871359.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hershey A, Chase M (1952). "Independent functions of viral protein and nucleic acid in growth of bacteriophage" (PDF). J Gen Physiol. 36 (1): 39–56. doi:10.1085/jgp.36.1.39. PMC 2147348. PMID 12981234.
- ^ The B-DNA X-ray pattern on the right of this linked image was obtained by Rosalind Franklin and Raymond Gosling in May 1952 at high hydration levels of DNA and it has been labeled as "Photo 51"
- ^ Nature Archives Double Helix of DNA: 50 Years
- ^ Original X-ray diffraction image
- ^ The Nobel Prize in Physiology or Medicine 1962 Nobelprize .org Accessed 22 December 06
- ^ Brenda Maddox (23 January 2003). "The double helix and the 'wronged heroine'" (PDF). Nature. 421 (6921): 407–408. doi:10.1038/nature01399. PMID 12540909. S2CID 4428347.
- ^ Crick, F.H.C. On degenerate templates and the adaptor hypothesis (PDF). genome.wellcome.ac.uk (Lecture, 1955). Accessed 22 December 2006
- ^ Meselson M, Stahl F (1958). "The replication of DNA in Escherichia coli". Proc Natl Acad Sci USA. 44 (7): 671–82. doi:10.1073/pnas.44.7.671. PMC 528642. PMID 16590258.
- ^ The Nobel Prize in Physiology or Medicine 1968 Nobelprize.org Accessed 22 December 06
Further reading
[edit]- Calladine, Chris R.; Drew, Horace R.; Luisi, Ben F. and Travers, Andrew A. (2003). Understanding DNA: the molecule & how it works. Amsterdam: Elsevier Academic Press. ISBN 0-12-155089-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Dennis, Carina; Julie Clayton (2003). 50 years of DNA. Basingstoke: Palgrave Macmillan. ISBN 1-4039-1479-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Judson, Horace Freeland (1996). The eighth day of creation: makers of the revolution in biology. Plainview, N.Y: CSHL Press. ISBN 0-87969-478-5.
- Olby, Robert C. (1994). The path to the double helix: the discovery of DNA. New York: Dover Publications. ISBN 0-486-68117-3., first published in October 1974 by MacMillan, with foreword by Francis Crick;the definitive DNA textbook,revised in 1994 with a 9 page postscript.
- Olby, Robert C. (2009). Francis Crick: A Biography. Plainview, N.Y: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-798-3.
- Ridley, Matt (2006). Francis Crick: discoverer of the genetic code. [Ashland, OH: Eminent Lives, Atlas Books. ISBN 0-06-082333-X.
- Berry, Andrew; Watson, James D. (2003). DNA: the secret of life. New York: Alfred A. Knopf. ISBN 0-375-41546-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Stent, Gunther Siegmund; Watson, James D. (1980). The double helix: a personal account of the discovery of the structure of DNA. New York: Norton. ISBN 0-393-95075-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Wilkins, Maurice (2003). The third man of the double helix the autobiography of Maurice Wilkins. Cambridge, Eng: University Press. ISBN 0-19-860665-6.
External links
[edit]- ‹The template Curlie is being considered for deletion.› DNA at Curlie
- DNA binding site prediction on protein
- DNA coiling to form chromosomes
- DNA from the Beginning Another DNA Learning Center site on DNA, genes, and heredity from Mendel to the human genome project.
- DNA Lab, demonstrates how to extract DNA from wheat using readily available equipment and supplies.
- DNA the Double Helix Game From the official Nobel Prize web site
- DNA under electron microscope
- Dolan DNA Learning Center
- Double Jelix: 50 years of DNA, Nature
- Double Helix 1953–2003 National Centre for Biotechnology Education
- Francis Crick and James Watson talking on the BBC in 1962, 1972, and 1974
- Genetic Education Modules for Teachers — DNA from the Beginning Study Guide
- Guide to DNA cloning
- Olby R (January 2003). "Quiet debut for the double helix" (PDF). Nature. 421 (6921): 402–5. doi:10.1038/nature01397. PMID 12540907. S2CID 4315521.
{{cite journal}}: CS1 maint: date and year (link) - PDB Molecule of the Month Bci2/Books/Wk4
- Rosalind Franklin's contributions to the study of DNA
- The Register of Francis Crick Personal Papers 1938 - 2007 at Mandeville Special Collections Library, Geisel Library, University of California, San Diego
- The Secret Life of DNA - DNA Music compositions
- U.S. National DNA Day — watch videos and participate in real-time chat with top scientists
- "Clue to chemistry of heredity found" (PDF). The New York Times. Saturday, June 13, 1953.
{{cite news}}: Check date values in:|date=(help) The first American newspaper coverage of the discovery of the DNA structure.
[[Category:DNA| ]] [[Category:Genetics]] [[Category:Helices]] [[Category:Diffraction]] [[Category:Scattering]]
- DNA molecular modeling
Molecular models of DNA structures are representations of the molecular geometry and topology of Deoxyribonucleic acid (DNA) molecules using one of several means, such as: closely packed spheres (CPK models) made of plastic, metal wires for 'skeletal models', graphic computations and animations by computers, artistic rendering, and so on, with the aim of simplifying and presenting the essential, physical and chemical, properties of DNA molecular structures either in vivo or in vitro. Computer molecular models also allow animations and molecular dynamics simulations that are very important for understanding how DNA functions in vivo. Thus, an old standing dynamic problem is how DNA "self-replication" takes place in living cells that should involve transient uncoiling of supercoiled DNA fibers. Although DNA consists of relatively rigid, very large elongated biopolymer molecules called "fibers" or chains (that are made of repeating nucleotide units of four basic types, attached to deoxyribose and phosphate groups), its molecular structure in vivo undergoes dynamic configuration changes that involve dynamically attached water molecules and ions. Supercoiling, packing with histones in chromosome structures, and other such supramolecular aspects also involve in vivo DNA topology which is even more complex than DNA molecular geometry, thus turning molecular modeling of DNA into an especially challenging problem for both molecular biologists and biotechnologists. Like other large molecules and biopolymers, DNA often exists in multiple stable geometries (that is, it exhibits conformational isomerism) and configurational, quantum states which are close to each other in energy on the potential energy surface of the DNA molecule. Such geometries can also be computed, at least in principle, by employing ab initio quantum chemistry methods that have high accuracy for small molecules. Such quantum geometries define an important class of ab initio molecular models of DNA whose exploration has barely started.

In an interesting twist of roles, the DNA molecule itself was proposed to be utilized for quantum computing. Both DNA nanostructures as well as DNA 'computing' biochips have been built (see biochip image at right).
The more advanced, computer-based molecular models of DNA involve molecular dynamics simulations as well as quantum mechanical computations of vibro-rotations, delocalized molecular orbitals (MOs), electric dipole moments, hydrogen-bonding, and so on.

Importance
[edit]From the very early stages of structural studies of DNA by X-ray diffraction and biochemical means, molecular models such as the Watson-Crick double-helix model were successfully employed to solve the 'puzzle' of DNA structure, and also find how the latter relates to its key functions in living cells. The first high quality X-ray diffraction patterns of A-DNA were reported by Rosalind Franklin and Raymond Gosling in 1953[1]. The first calculations of the Fourier transform of an atomic helix were reported one year earlier by Cochran, Crick and Vand [2], and were followed in 1953 by the computation of the Fourier transform of a coiled-coil by Crick[3]. The first reports of a double-helix molecular model of B-DNA structure were made by Watson and Crick in 1953[4][5]. Last-but-not-least, Maurice F. Wilkins, A. Stokes and H.R. Wilson, reported the first X-ray patterns of in vivo B-DNA in partially oriented salmon sperm heads [6]. The development of the first correct double-helix molecular model of DNA by Crick and Watson may not have been possible without the biochemical evidence for the nucleotide base-pairing ([A---T]; [C---G]), or Chargaff's rules[7][8][9][10][11][12].
Examples of DNA molecular models
[edit]Animated molecular models allow one to visually explore the three-dimensional (3D) structure of DNA. The first DNA model is a space-filling, or CPK, model of the DNA double-helix whereas the third is an animated wire, or skeletal type, molecular model of DNA. The last two DNA molecular models in this series depict quadruplex DNA that may be involved in certain cancers[13][14]. The last figure on this panel is a molecular model of hydrogen bonds between water molecules in ice that are similar to those found in DNA.
- Spacefilling models or CPK models - a molecule is represented by overlapping spheres representing the atoms.

Images for DNA Structure Determination from X-Ray Patterns
[edit]The following images illustrate both the principles and the main steps involved in generating structural information from X-ray diffraction studies of oriented DNA fibers with the help of molecular models of DNA that are combined with crystallographic and mathematical analysis of the X-ray patterns. From left to right the gallery of images shows:
- First row:
- 1. Constructive X-ray interference, or diffraction, following Bragg's Law of X-ray "reflection by the crystal planes";
- 2. A comparison of A-DNA (crystalline) and highly hydrated B-DNA (paracrystalline) X-ray diffraction, and respectively, X-ray scattering patterns (courtesy of Dr. Herbert R. Wilson, FRS- see refs. list);
- 3. Purified DNA precipitated in a water jug;
- 4. The major steps involved in DNA structure determination by X-ray crystallography showing the important role played by molecular models of DNA structure in this iterative, structure--determination process;
- Second row:
- 5. Photo of a modern X-ray diffractometer employed for recording X-ray patterns of DNA with major components: X-ray source, goniometer, sample holder, X-ray detector and/or plate holder;
- 6. Illustrated animation of an X-ray goniometer;
- 7. X-ray detector at the SLAC synchrotron facility;
- 8. Neutron scattering facility at ISIS in UK;
- Third and fourth rows: Molecular models of DNA structure at various scales; figure #11 is an actual electron micrograph of a DNA fiber bundle, presumably of a single bacterial chromosome loop.
Paracrystalline lattice models of B-DNA structures
[edit]A paracrystalline lattice, or paracrystal, is a molecular or atomic lattice with significant amounts (e.g., larger than a few percent) of partial disordering of molecular arranegements. Limiting cases of the paracrystal model are nanostructures, such as glasses, liquids, etc., that may possess only local ordering and no global order. Liquid crystals also have paracrystalline rather than crystalline structures.
thumb|500px|center|DNA Helix controversy in 1952
Highly hydrated B-DNA occurs naturally in living cells in such a paracrystalline state, which is a dynamic one in spite of the relatively rigid DNA double-helix stabilized by parallel hydrogen bonds between the nucleotide base-pairs in the two complementary, helical DNA chains (see figures). For simplicity most DNA molecular models ommit both water and ions dynamically bound to B-DNA, and are thus less useful for understanding the dynamic behaviors of B-DNA in vivo. The physical and mathematical analysis of X-ray[15][16] and spectroscopic data for paracrystalline B-DNA is therefore much more complicated than that of crystalline, A-DNA X-ray diffraction patterns. The paracrystal model is also important for DNA technological applications such as DNA nanotechnology. Novel techniques that combine X-ray diffraction of DNA with X-ray microscopy in hydrated living cells are now also being developed (see, for example, "Application of X-ray microscopy in the analysis of living hydrated cells").
Genomic and Biotechnology Applications of DNA molecular modeling
[edit]The following gallery of images illustrates various uses of DNA molecular modeling in Genomics and Biotechnology research applications from DNA repair to PCR and DNA nanostructures; each slide contains its own explanation and/or details. The first slide presents an overview of DNA applications, including DNA molecular models, with emphasis on Genomics and Biotechnology.
Gallery: DNA Molecular modeling applications
[edit]- NDB ID: UD0017 Database
- X-ray Atlas -database
- PDB files of coordinates for nucleic acid structures from X-ray diffraction by NA (incl. DNA) crystals
- Structure factors dowloadable files in CIF format
- [[Neutron scattering]
- ISIS neutron source
- ISIS pulsed neutron source:A world centre for science with neutrons & muons at Harwell, near Oxford, UK.
Two-dimensional DNA junction arrays have been visualized by Atomic Force Microscopy (AFM)[17]. Other imaging resources for AFM/Scanning probe microscopy(SPM) can be freely accessed at:
Gallery of AFM Images
[edit]Mass spectrometry--Maldi informatics
[edit]Spectroscopy
[edit]- NIR-FT[21][22][23]
- Spectral,
- Hyperspectral, and
- Chemical imaging)[24][25][26][27][28][29][30].
- Raman spectroscopy/microscopy[31] and CARS[32].
- Fluorescence correlation spectroscopy[33][34][35][36][37][38][39][40], Fluorescence cross-correlation spectroscopy and FRET[41][42][43].
Gallery: CARS (Raman spectroscopy), Fluorescence confocal microscopy, and Hyperspectral imaging
[edit]Genomic and structural databases
[edit]- CBS Genome Atlas Database — contains examples of base skews.[45]
- The Z curve database of genomes — a 3-dimensional visualization and analysis tool of genomes[46].
- DNA and other nucleic acids' molecular models: Coordinate files of nucleic acids molecular structure models in PDB and CIF formats
Notes
[edit]- ^ Franklin, R.E. and Gosling, R.G. recd.6 March 1953. Acta Cryst. (1953). 6, 673 The Structure of Sodium Thymonucleate Fibres I. The Influence of Water Content Acta Cryst. (1953). and 6, 678 The Structure of Sodium Thymonucleate Fibres II. The Cylindrically Symmetrical Patterson Function.
- ^ Cochran, W., Crick, F.H.C. and Vand V. 1952. The Structure of Synthetic Polypeptides. 1. The Transform of Atoms on a Helix. Acta Cryst. 5(5):581-586.
- ^ Crick, F.H.C. 1953a. The Fourier Transform of a Coiled-Coil., Acta Crystallographica 6(8-9):685-689.
- ^ Watson, J.D; Crick F.H.C. 1953a. Molecular Structure of Nucleic Acids- A Structure for Deoxyribose Nucleic Acid., Nature 171(4356):737-738.
- ^ Watson, J.D; Crick F.H.C. 1953b. The Structure of DNA., Cold Spring Harbor Symposia on Qunatitative Biology 18:123-131.
- ^ Wilkins M.H.F., A.R. Stokes A.R. & Wilson, H.R. (1953). "Molecular Structure of Deoxypentose Nucleic Acids" (PDF). Nature. 171 (4356): 738–740. doi:10.1038/171738a0. PMID 13054693. S2CID 4280080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Elson D, Chargaff E (1952). "On the deoxyribonucleic acid content of sea urchin gametes". Experientia. 8 (4): 143–145. doi:10.1007/BF02170221. PMID 14945441. S2CID 36803326.
- ^ Chargaff E, Lipshitz R, Green C (1952). "Composition of the deoxypentose nucleic acids of four genera of sea-urchin". J Biol Chem. 195 (1): 155–160. doi:10.1016/S0021-9258(19)50884-5. PMID 14938364.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chargaff E, Lipshitz R, Green C, Hodes ME (1951). "The composition of the deoxyribonucleic acid of salmon sperm". J Biol Chem. 192 (1): 223–230. doi:10.1016/S0021-9258(18)55924-X. PMID 14917668.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chargaff E (1951). "Some recent studies on the composition and structure of nucleic acids". J Cell Physiol Suppl. 38 (Suppl).
- ^ Magasanik B, Vischer E, Doniger R, Elson D, Chargaff E (1950). "The separation and estimation of ribonucleotides in minute quantities". J Biol Chem. 186 (1): 37–50. doi:10.1016/S0021-9258(18)56284-0. PMID 14778802.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chargaff E (1950). "Chemical specificity of nucleic acids and mechanism of their enzymatic degradation". Experientia. 6 (6): 201–209. doi:10.1007/BF02173653. PMID 15421335. S2CID 2522535.
- ^ http://www.phy.cam.ac.uk/research/bss/molbiophysics.php
- ^ http://planetphysics.org/encyclopedia/TheoreticalBiophysics.html
- ^ Hosemann R., Bagchi R.N., Direct analysis of diffraction by matter, North-Holland Publs., Amsterdam – New York, 1962.
- ^ Baianu, I.C. (1978). "X-ray scattering by partially disordered membrane systems". Acta Cryst. A34 (5): 751–753. doi:10.1107/S0567739478001540.
- ^ Mao, Chengde; Sun, Weiqiong; Seeman, Nadrian C. (1999). "Designed Two-Dimensional DNA Holliday Junction Arrays Visualized by Atomic Force Microscopy". Journal of the American Chemical Society. 121 (23): 5437–5443. doi:10.1021/ja9900398. ISSN 0002-7863.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ http://www.jonathanpmiller.com/Karplus.html- obtaining dihedral angles from 3J coupling constants
- ^ http://www.spectroscopynow.com/FCKeditor/UserFiles/File/specNOW/HTML%20files/General_Karplus_Calculator.htm Another Javascript-like NMR coupling constant to dihedral
- ^ Lee, S. C. et al., (2001). One Micrometer Resolution NMR Microscopy. J. Magn. Res., 150: 207-213.
- ^ Near Infrared Microspectroscopy, Fluorescence Microspectroscopy,Infrared Chemical Imaging and High Resolution Nuclear Magnetic Resonance Analysis of Soybean Seeds, Somatic Embryos and Single Cells., Baianu, I.C. et al. 2004., In Oil Extraction and Analysis., D. Luthria, Editor pp.241-273, AOCS Press., Champaign, IL.
- ^ Single Cancer Cell Detection by Near Infrared Microspectroscopy, Infrared Chemical Imaging and Fluorescence Microspectroscopy.2004.I. C. Baianu, D. Costescu, N. E. Hofmann and S. S. Korban, q-bio/0407006 (July 2004)
- ^ Raghavachari, R., Editor. 2001. Near-Infrared Applications in Biotechnology, Marcel-Dekker, New York, NY.
- ^ http://www.imaging.net/chemical-imaging/ Chemical imaging
- ^ http://www.malvern.com/LabEng/products/sdi/bibliography/sdi_bibliography.htm E. N. Lewis, E. Lee and L. H. Kidder, Combining Imaging and Spectroscopy: Solving Problems with Near-Infrared Chemical Imaging. Microscopy Today, Volume 12, No. 6, 11/2004.
- ^ D.S. Mantus and G. H. Morrison. 1991. Chemical imaging in biology and medicine using ion microscopy., Microchimica Acta, 104, (1-6) January 1991, doi: 10.1007/BF01245536
- ^ Near Infrared Microspectroscopy, Fluorescence Microspectroscopy,Infrared Chemical Imaging and High Resolution Nuclear Magnetic Resonance Analysis of Soybean Seeds, Somatic Embryos and Single Cells., Baianu, I.C. et al. 2004., In Oil Extraction and Analysis., D. Luthria, Editor pp.241-273, AOCS Press., Champaign, IL.
- ^ Single Cancer Cell Detection by Near Infrared Microspectroscopy, Infrared Chemical Imaging and Fluorescence Microspectroscopy.2004.I. C. Baianu, D. Costescu, N. E. Hofmann and S. S. Korban, q-bio/0407006 (July 2004)
- ^ J. Dubois, G. Sando, E. N. Lewis, Near-Infrared Chemical Imaging, A Valuable Tool for the Pharmaceutical Industry, G.I.T. Laboratory Journal Europe, No.1-2, 2007.
- ^ Applications of Novel Techniques to Health Foods, Medical and Agricultural Biotechnology.(June 2004).,I. C. Baianu, P. R. Lozano, V. I. Prisecaru and H. C. Lin q-bio/0406047
- ^ Chemical Imaging Without Dyeing
- ^ C.L. Evans and X.S. Xie.2008. Coherent Anti-Stokes Raman Scattering Microscopy: Chemical Imaging for Biology and Medicine., doi:10.1146/annurev.anchem.1.031207.112754 Annual Review of Analytical Chemistry, 1: 883-909.
- ^ Eigen, M., Rigler, M. Sorting single molecules: application to diagnostics and evolutionary biotechnology,(1994) Proc. Natl. Acad. Sci. USA, 91,5740-5747.
- ^ Rigler, M. Fluorescence correlations, single molecule detection and large number screening. Applications in biotechnology,(1995) J. Biotechnol., 41,177-186.
- ^ Rigler R. and Widengren J. (1990). Ultrasensitive detection of single molecules by fluorescence correlation spectroscopy, BioScience (Ed. Klinge & Owman) p.180.
- ^ Single Cancer Cell Detection by Near Infrared Microspectroscopy, Infrared Chemical Imaging and Fluorescence Microspectroscopy.2004.I. C. Baianu, D. Costescu, N. E. Hofmann, S. S. Korban and et al., q-bio/0407006 (July 2004)
- ^ Oehlenschläger F., Schwille P. and Eigen M. (1996). Detection of HIV-1 RNA by nucleic acid sequence-based amplification combined with fluorescence correlation spectroscopy, Proc. Natl. Acad. Sci. USA 93:1281.
- ^ Bagatolli, L.A., and Gratton, E. (2000). Two-photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys J., 78:290-305.
- ^ Schwille, P., Haupts, U., Maiti, S., and Webb. W.(1999). Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophysical Journal, 77(10):2251-2265.
- ^ Near Infrared Microspectroscopy, Fluorescence Microspectroscopy,Infrared Chemical Imaging and High Resolution Nuclear Magnetic Resonance Analysis of Soybean Seeds, Somatic Embryos and Single Cells., Baianu, I.C. et al. 2004., In Oil Extraction and Analysis., D. Luthria, Editor pp.241-273, AOCS Press., Champaign, IL.
- ^ FRET description
- ^ doi:10.1016/S0959-440X(00)00190-1Recent advances in FRET: distance determination in protein–DNA complexes. Current Opinion in Structural Biology 2001, 11(2), 201-207
- ^ http://www.fretimaging.org/mcnamaraintro.html FRET imaging introduction
- ^ Eigen, M., and Rigler, R. (1994). Sorting single molecules: Applications to diagnostics and evolutionary biotechnology, Proc. Natl. Acad. Sci. USA 91:5740.
- ^ Hallin PF, David Ussery D (2004). "CBS Genome Atlas Database: A dynamic storage for bioinformatic results and DNA sequence data". Bioinformatics. 20 (18): 3682–3686. doi:10.1093/bioinformatics/bth423. PMID 15256401.
- ^ Zhang CT, Zhang R, Ou HY (2003). "The Z curve database: a graphic representation of genome sequences". Bioinformatics 19 (5): 593-599. doi:10.1093/bioinformatics/btg041
References
[edit]- Applications of Novel Techniques to Health Foods, Medical and Agricultural Biotechnology.(June 2004) I. C. Baianu, P. R. Lozano, V. I. Prisecaru and H. C. Lin., q-bio/0406047.
- F. Bessel, Untersuchung des Theils der planetarischen Störungen, Berlin Abhandlungen (1824), article 14.
- Sir Lawrence Bragg, FRS. The Crystalline State, A General survey. London: G. Bells and Sons, Ltd., vols. 1 and 2., 1966., 2024 pages.
- Cantor, C. R. and Schimmel, P.R. Biophysical Chemistry, Parts I and II., San Franscisco: W.H. Freeman and Co. 1980. 1,800 pages.
- Eigen, M., and Rigler, R. (1994). Sorting single molecules: Applications to diagnostics and evolutionary biotechnology, Proc. Natl. Acad. Sci. USA 91:5740.
- Raghavachari, R., Editor. 2001. Near-Infrared Applications in Biotechnology, Marcel-Dekker, New York, NY.
- Rigler R. and Widengren J. (1990). Ultrasensitive detection of single molecules by fluorescence correlation spectroscopy, BioScience (Ed. Klinge & Owman) p.180.
- Single Cancer Cell Detection by Near Infrared Microspectroscopy, Infrared Chemical Imaging and Fluorescence Microspectroscopy.2004. I. C. Baianu, D. Costescu, N. E. Hofmann, S. S. Korban and et al., q-bio/0407006 (July 2004).
- Voet, D. and J.G. Voet. Biochemistry, 2nd Edn., New York, Toronto, Singapore: John Wiley & Sons, Inc., 1995, ISBN: 0-471-58651-X., 1361 pages.
- Watson, G. N. A Treatise on the Theory of Bessel Functions., (1995) Cambridge University Press. ISBN 0-521-48391-3.
- Watson, James D. and Francis H.C. Crick. A structure for Deoxyribose Nucleic Acid (PDF). Nature 171, 737–738, 25 April 1953.
- Watson, James D. Molecular Biology of the Gene. New York and Amsterdam: W.A. Benjamin, Inc. 1965., 494 pages.
- Wentworth, W.E. Physical Chemistry. A short course., Malden (Mass.): Blackwell Science, Inc. 2000.
- Herbert R. Wilson, FRS. Diffraction of X-rays by proteins, Nucleic Acids and Viruses., London: Edward Arnold (Publishers) Ltd. 1966.
- Kurt Wuthrich. NMR of Proteins and Nucleic Acids., New York, Brisbane,Chicester, Toronto, Singapore: J. Wiley & Sons. 1986., 292 pages.
- Robinson, Bruche H. (August 1987). "The Design of a Biochip: A Self-Assembling Molecular-Scale Memory Device". Protein Engineering. 1 (4): 295–300. doi:10.1093/protein/1.4.295. ISSN 0269-2139. PMID 3508280.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) Link - Rothemund, Paul W. K.; Ekani-Nkodo, Axel; Papadakis, Nick; Kumar, Ashish; Fygenson, Deborah Kuchnir; Winfree, Erik (2004). "Design and Characterization of Programmable DNA Nanotubes". Journal of the American Chemical Society. 126 (50): 16344–16352. doi:10.1021/ja044319l. ISSN 0002-7863. PMID 15600335.
{{cite journal}}: Unknown parameter|month=ignored (help) - Keren, Kinneret; Berman, Rotem S.; Buchstab, Evgeny; Sivan, Uri; Braun, Erez (November 2003). "DNA-Templated Carbon Nanotube Field-Effect Transistor". Science. 302 (6549): 1380–1382. doi:10.1126/science.1091022. ISSN 1095-9203. PMID 14631035. S2CID 29794525.
{{cite journal}}: CS1 maint: date and year (link) - Zheng, Jiwen (2006). "2D Nanoparticle Arrays Show the Organizational Power of Robust DNA Motifs". Nano Letters. 6 (7): 1502–1504. doi:10.1021/nl060994c. ISSN 1530-6984. PMC 3465979. PMID 16834438.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Cohen, Justin D.; Sadowski, John P.; Dervan, Peter B. (2007). "Addressing Single Molecules on DNA Nanostructures". Angewandte Chemie. 46 (42): 7956–7959. doi:10.1002/anie.200702767. ISSN 0570-0833. PMID 17763481.
- Mao, Chengde; Sun, Weiqiong; Seeman, Nadrian C. (1999). "Designed Two-Dimensional DNA Holliday Junction Arrays Visualized by Atomic Force Microscopy". Journal of the American Chemical Society. 121 (23): 5437–5443. doi:10.1021/ja9900398. ISSN 0002-7863.
{{cite journal}}: Unknown parameter|month=ignored (help) - Constantinou, Pamela E.; Wang, Tong; Kopatsch, Jens; Israel, Lisa B.; Zhang, Xiaoping; Ding, Baoquan; Sherman, William B.; Wang, Xing; Zheng, Jianping; Sha, Ruojie; Seeman, Nadrian C. (2006). "Double cohesion in structural DNA nanotechnology". Organic and Biomolecular Chemistry. 4 (18): 3414–3419. doi:10.1039/b605212f. PMC 3491902. PMID 17036134.
DNA-Advanced Techniques wk4v5
[edit]- NMR Spectroscopy
- Microwave spectroscopy
- Two-dimensional IR spectroscopy
- X-ray scattering
- Spectral imaging
- Hyperspectral imaging
- Chemical imaging
- NMR microscopy
- VCD or Vibrational circular dichroism
- Fluorescence cross-correlation spectroscopy (FCCS)
- Molecular structure
- Molecular geometry
- Molecular topology
- DNA structure
- DNA Dynamics
- DNA nanotechnology
- Imaging
- Atomic force microscope
- Electron microscope
- X-ray microscope
- Sirius visualization software
- Nanostructure
- Liquid crystal
- Glasses
- QMC@Home
- Sir Lawrence Bragg, FRS
- Sir John Randall
- James Watson
- Francis Crick
- Maurice Wilkins
- Herbert Wilson, FRS
- Alex Stokes
Version 5 of 34 Mb, 245 pages
[edit]- DNA the Double Helix Game From the official Nobel Prize web site
- MDDNA: Structural Bioinformatics of DNA
- Double Helix 1953–2003 National Centre for Biotechnology Education
- DNA under electron microscope
- Ascalaph DNA — Commercial software for DNA modeling
- DNAlive: a web interface to compute DNA physical properties. Also allows cross-linking of the results with the UCSC Genome browser and DNA dynamics.
- DiProDB: Dinucleotide Property Database. The database is designed to collect and analyse thermodynamic, structural and other dinucleotide properties.
- Further details of mathematical and molecular analysis of DNA structure based on X-ray data
- Bessel functions corresponding to Fourier transforms of atomic or molecular helices.
- Application of X-ray microscopy in analysis of living hydrated cells
- Characterization in nanotechnology some pdfs
- overview of STM/AFM/SNOM principles with educative videos
- SPM Image Gallery - AFM STM SEM MFM NSOM and More
- How SPM Works
- U.S. National DNA Day — watch videos and participate in real-time discusssions with scientists.
- The Secret Life of DNA - DNA Music compositions
[[Category:DNA| ]] [[Category:Molecular geometry]] [[Category:Helices]] [[Category:Diffraction]] [[Category:Molecular dynamics]] [[Category:Scattering]] [[Category:Quantum chemistry]] [[Category:Computational models]] [[Category:Molecular biology]] [[Category:Molecular genetics]] [[Category:Genomics]] [[Category:Genetics]] [[Category:Crystallography]] [[Category:Spectroscopy]] [[Category:Quantum information science| ]] [[Category:Computational complexity theory|*]] [[Category:Polymers]] [[Category:Biomolecules]] [[Category:Nanotechnology]] [[Category:Biotechnology products]] [[Category:Databases]] [[Category:Imaging]] [[Category:Microscopic images]] [[Category:Scanning probe microscopy]] [[Category:Fluorescence]] [[Category:Database management systems]] [[Category:Classes of computers]] [[Category:Information theory]] [[Category:Nobel laureates in Physiology or Medicine]] [[Category:Molecular biologists]] [[Category:Biophysicists]]