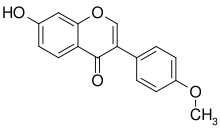
Formononetin
Appearance
| |

| |
| Names | |
|---|---|
| IUPAC name
7-Hydroxy-4′-methoxyisoflavone
| |
| Systematic IUPAC name
7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Biochanin B
Formononetol 4'-O-methyldaidzein | |
| Identifiers | |
3D model (JSmol)
|
|
| 237979 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.931 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O4 | |
| Molar mass | 268.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Formononetin is an O-methylated isoflavone.
Natural occurrences
[edit]Formononetin is found in a number of plants and herbs such as red clover.[1] Along with other phytoestrogens, it predominantly occurs in leguminous plants and Fabaceae, particularly in beans, such as green beans, lima beans, soy and many others, as the free aglycone or in form of its glucoside ononin.[2]
It can also be found in Maackia amurensis cell cultures.[3]
Pharmacodynamics
[edit]Formononetin promotes angiogenesis.[4] It is also involved in expressing the gene and proteins that are needed to make IgE.[5]
Derivatives
[edit]Ononin is the 7-O-β-D-glucopyranoside of formononetin.[6]
References
[edit]- ^ Medjakovic, S.; Jungbauer, A. (2008). "Red Clover Isoflavones Biochanin A and Formononetin are Potent Ligands of the Human Aryl Hydrocarbon Receptor". The Journal of Steroid Biochemistry and Molecular Biology. 108 (1–2): 171–177. doi:10.1016/j.jsbmb.2007.10.001. PMID 18060767. S2CID 206495959.
- ^ "Iowa State University Database on the Isoflavone Content of Foods, Release 1.3". USDA. 2002. Archived from the original on 2012-11-02. Retrieved 2011-02-13.
- ^ Fedoreyev, S.A; Pokushalova, T.V; Veselova, M.V; Glebko, L.I; Kulesh, N.I; Muzarok, T.I; Seletskaya, L.D; Bulgakov, V.P; Zhuravlev, Yu.N (2000). "Isoflavonoid production by callus cultures of Maackia amurensis". Fitoterapia. 71 (4): 365–72. doi:10.1016/S0367-326X(00)00129-5. PMID 10925005.
- ^ Li, Shang; Dang, Yuanye; Zhou, Xuelin; Huang, Bin; Huang, Xiaohui; Zhang, Zherui; Kwan, Yiu Wa; Chan, Shun Wan; Leung, George Pak Heng; Lee, Simon Ming Yuen; Hoi, Maggie Pui Man (2015). "Formononetin promotes angiogenesis through the estrogen receptor alpha-enhanced ROCK pathway". Scientific Reports. 5: 16815. Bibcode:2015NatSR...516815L. doi:10.1038/srep16815. PMC 4645220. PMID 26568398.
- ^ "This plant-based compound could finally cure food allergies". ZME Science. 2022-04-01. Retrieved 2022-04-04.
- ^ You-Ping Zhu (1998-05-28). Chinese Materia Medica: Chemistry, Pharmacology and Applications. CRC Press. p. 622. ISBN 9057022850.
