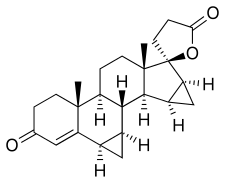
Drospirenone
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Droe-SPY-re-nown |
| Trade names | Alone: Slynd With estradiol: Angeliq With ethinylestradiol: Yasmin, Yasminelle, Yaz, others With estetrol: Nextstellis |
| Other names | Dihydrospirenone; Dihydrospirorenone; 1,2-Dihydrospirorenone; MSp; SH-470; ZK-30595; LF-111; 17β-Hydroxy-6β,7β:15β,16β-dimethylene-3-oxo-17α-pregn-4-ene-21-carboxylic acid, γ-lactone |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin; Antimineralocorticoid; Steroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 66–85%[1][4][5] |
| Protein binding | 95–97% (to albumin)[3][1][4] |
| Metabolism | Liver (mostly CYP450-independent (reduction, sulfation, and cleavage of lactone ring), some CYP3A4 contribution)[4][6][7][8] |
| Metabolites | • Drospirenone acid[3] • 4,5-Dihydrodrospirenone 3-sulfate[3] |
| Elimination half-life | 25–33 hours[3][4][1] |
| Excretion | Urine, feces[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.060.599 |
| Chemical and physical data | |
| Formula | C24H30O3 |
| Molar mass | 366.501 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses.[1][9] It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others.[9][3] The medication is an analog of the drug spironolactone.[10] Drospirenone is taken by mouth.[1][3]
Common side effects include acne, headache, breast tenderness, weight increase, and menstrual changes.[3] Rare side effects may include high potassium levels and blood clots (when taken as a combined oestrogen-progestogen pill), among others.[3][11] Drospirenone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It has additional antimineralocorticoid and antiandrogenic activity and no other important hormonal activity.[1] Because of its antimineralocorticoid activity and lack of undesirable off-target activity, drospirenone is said to more closely resemble bioidentical progesterone than other progestins.[12][13]
Drospirenone was patented in 1976 and introduced for medical use in 2000.[14][15] It is available widely throughout the world.[9] The medication is sometimes referred to as a "fourth-generation" progestin.[16][17] It is available as a generic medication.[18] In 2020, a formulation of drospirenone with ethinylestradiol was the 145th most commonly prescribed medication in the United States, with more than 4 million prescriptions.[19][20]
Medical uses
[edit]Drospirenone (DRSP) is used by itself as a progestogen-only birth control pill, in combination with the estrogens ethinylestradiol (EE) or estetrol (E4), with or without supplemental folic acid (vitamin B9), as a combined birth control pill, and in combination with the estrogen estradiol (E2) for use in menopausal hormone therapy.[3] A birth control pill with low-dose ethinylestradiol is also indicated for the treatment of moderate acne, premenstrual syndrome (PMS), premenstrual dysphoric disorder (PMDD), and dysmenorrhea (painful menstruation).[21][22] For use in menopausal hormone therapy, E2/DRSP is specifically approved to treat moderate to severe vasomotor symptoms (hot flashes), vaginal atrophy, and postmenopausal osteoporosis.[23][24][25] The drospirenone component in this formulation is included specifically to prevent estrogen-induced endometrial hyperplasia.[26] Drospirenone has also been used in combination with an estrogen as a component of hormone therapy for transgender women.[27][28]
Studies have found that EE/DRSP is superior to placebo in reducing premenstrual emotional and physical symptoms while also improving quality of life.[29][30] E2/DRSP has been found to increase bone mineral density and to reduce the occurrence of bone fractures in postmenopausal women.[31][26][32][33] In addition, E2/DRSP has a favorable influence on cholesterol and triglyceride levels and decreases blood pressure in women with high blood pressure.[32][33] Due to its antimineralocorticoid activity, drospirenone opposes estrogen-induced salt and water retention and maintains or slightly reduces body weight.[34]
Available forms
[edit]Drospirenone is available in the following formulations, brand names, and indications:[35][36]
- Drospirenone 4 mg (Slynd) – progestogen-only birth control pill[3]
- Drospirenone 3 mg and estetrol 14.2 mg (Nextstellis (US)) – combined birth control pill[37][38][39]
- Ethinylestradiol 30 μg and drospirenone 3 mg (Ocella, Syeda, Yasmin, Zarah, Zumandimine) – combined birth control pill[40][41][42][43]
- Ethinylestradiol 20 μg and drospirenone 3 mg (Gianvi, Jasmiel, Loryna, Lo-Zumandimine, Nikki, Vestura, Yaz) – combined birth control pill, acne, PMS, PMDD, dysmenorrhea[21]
- Ethinylestradiol 30 μg, drospirenone 3 mg, and levomefolate calcium 0.451 mg (Beyaz, Tydemy) – combined birth control pill with vitamin B9 supplementation, acne, PMS[44][45]
- Estetrol 15 mg and drospirenone 3 mg (Nextstellis (CA)) – combined birth control pill[46][47]
- Estradiol 0.5 or 1 mg and drospirenone 0.25 or 0.5 mg (Angeliq) – menopausal hormone therapy (menopausal syndrome, postmenopausal osteoporosis)[23]
Contraindications
[edit]Contraindications of drospirenone include renal impairment or chronic kidney disease, adrenal insufficiency, presence or history of cervical cancer or other progestogen-sensitive cancers, benign or malignant liver tumors or hepatic impairment, undiagnosed abnormal uterine bleeding, and hyperkalemia (high potassium levels).[3][48][49] Renal impairment, hepatic impairment, and adrenal insufficiency are contraindicated because they increase exposure to drospirenone and/or increase the risk of hyperkalemia with drospirenone.[3]
Side effects
[edit]Adverse effects of drospirenone alone occurring in more than 1% of women may include unscheduled menstrual bleeding (breakthrough or intracyclic) (40.3–64.4%), acne (3.8%), metrorrhagia (2.8%), headache (2.7%), breast pain (2.2%), weight gain (1.9%), dysmenorrhea (1.9%), nausea (1.8%), vaginal hemorrhage (1.7%), decreased libido (1.3%), breast tenderness (1.2%), and irregular menstruation (1.2%).[3]
High potassium levels
[edit]Drospirenone is an antimineralocorticoid with potassium-sparing properties, though in most cases no increase of potassium levels is to be expected.[48] In women with mild or moderate chronic kidney disease, or in combination with chronic daily use of other potassium-sparing medications (ACE inhibitors, angiotensin II receptor antagonists, potassium-sparing diuretics, heparin, antimineralocorticoids, or nonsteroidal anti-inflammatory drugs), a potassium level should be checked after two weeks of use to test for hyperkalemia.[48][50] Persistent hyperkalemia that required discontinuation occurred in 2 out of around 1,000 women (0.2%) with 4 mg/day drospirenone alone in clinical trials.[3]
Blood clots
[edit]Birth control pills containing ethinylestradiol and a progestin are associated with an increased risk of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE).[51] The incidence is about 4-fold higher on average than in women not taking a birth control pill.[51] The absolute risk of VTE with ethinylestradiol-containing birth control pills is small, in the area of 3 to 10 out of 10,000 women per year, relative to 1 to 5 out of 10,000 women per year not taking a birth control pill.[52][53] The risk of VTE during pregnancy is 5 to 20 in 10,000 women per year and during the postpartum period is 40 to 65 per 10,000 women per year.[53] The higher risk of VTE with combined birth control pills is thought to be due to the ethinylestradiol component, as ethinylestradiol has estrogenic effects on liver synthesis of coagulation factors which result in a procoagulatory state.[11] In contrast to ethinylestradiol-containing birth control pills, neither progestogen-only birth control nor the combination of transdermal estradiol and an oral progestin in menopausal hormone therapy is associated with an increased risk of VTE.[11][54]
Different progestins in ethinylestradiol-containing birth control pills have been associated with different risks of VTE.[11] Birth control pills containing progestins such as desogestrel, gestodene, drospirenone, and cyproterone acetate have been found to have 2- to 3-fold the risk of VTE of birth control pills containing levonorgestrel in retrospective cohort and nested case–control observational studies.[11][52] However, this area of research is controversial, and confounding factors may have been present in these studies.[11][52][55] Other observational studies, specifically prospective cohort and case control studies, have found no differences in risk between different progestins, including between birth control pills containing drospirenone and birth control pills containing levonorgestrel.[11][52][55][56] These kinds of observational studies have certain advantages over the aforementioned types of studies, like better ability to control for confounding factors.[56] Systematic reviews and meta-analyses of all of the data in the mid-to-late 2010s found that birth control pills containing cyproterone acetate, desogestrel, drospirenone, or gestodene overall were associated with a risk of VTE of about 1.3- to 2.0-fold compared to that of levonorgestrel-containing birth control pills.[57][58][52]
Androgenic progestins have been found to antagonize to some degree the effects of ethinylestradiol on coagulation.[59][60][61][62] As a result, more androgenic progestins, like levonorgestrel and norethisterone, may oppose the procoagulatory effects of ethinylestradiol and result in a lower increase in risk of VTE.[11][63] Conversely, this would be the case less or not at all with progestins that are less androgenic, like desogestrel and gestodene, as well as with progestins that are antiandrogenic, like drospirenone and cyproterone acetate.[11][63]
In the early 2010s, the FDA updated the label for birth control pills containing drospirenone and other progestins to include warnings for stopping use prior to and after surgery, and to warn that such birth control pills may have a higher risk of blood clots.[49]
| Type | Route | Medications | Odds ratio (95% CI) |
|---|---|---|---|
| Menopausal hormone therapy | Oral | Estradiol alone ≤1 mg/day >1 mg/day |
1.27 (1.16–1.39)* 1.22 (1.09–1.37)* 1.35 (1.18–1.55)* |
| Conjugated estrogens alone ≤0.625 mg/day >0.625 mg/day |
1.49 (1.39–1.60)* 1.40 (1.28–1.53)* 1.71 (1.51–1.93)* | ||
| Estradiol/medroxyprogesterone acetate | 1.44 (1.09–1.89)* | ||
| Estradiol/dydrogesterone ≤1 mg/day E2 >1 mg/day E2 |
1.18 (0.98–1.42) 1.12 (0.90–1.40) 1.34 (0.94–1.90) | ||
| Estradiol/norethisterone ≤1 mg/day E2 >1 mg/day E2 |
1.68 (1.57–1.80)* 1.38 (1.23–1.56)* 1.84 (1.69–2.00)* | ||
| Estradiol/norgestrel or estradiol/drospirenone | 1.42 (1.00–2.03) | ||
| Conjugated estrogens/medroxyprogesterone acetate | 2.10 (1.92–2.31)* | ||
| Conjugated estrogens/norgestrel ≤0.625 mg/day CEEs >0.625 mg/day CEEs |
1.73 (1.57–1.91)* 1.53 (1.36–1.72)* 2.38 (1.99–2.85)* | ||
| Tibolone alone | 1.02 (0.90–1.15) | ||
| Raloxifene alone | 1.49 (1.24–1.79)* | ||
| Transdermal | Estradiol alone ≤50 μg/day >50 μg/day |
0.96 (0.88–1.04) 0.94 (0.85–1.03) 1.05 (0.88–1.24) | |
| Estradiol/progestogen | 0.88 (0.73–1.01) | ||
| Vaginal | Estradiol alone | 0.84 (0.73–0.97) | |
| Conjugated estrogens alone | 1.04 (0.76–1.43) | ||
| Combined birth control | Oral | Ethinylestradiol/norethisterone | 2.56 (2.15–3.06)* |
| Ethinylestradiol/levonorgestrel | 2.38 (2.18–2.59)* | ||
| Ethinylestradiol/norgestimate | 2.53 (2.17–2.96)* | ||
| Ethinylestradiol/desogestrel | 4.28 (3.66–5.01)* | ||
| Ethinylestradiol/gestodene | 3.64 (3.00–4.43)* | ||
| Ethinylestradiol/drospirenone | 4.12 (3.43–4.96)* | ||
| Ethinylestradiol/cyproterone acetate | 4.27 (3.57–5.11)* | ||
| Notes: (1) Nested case–control studies (2015, 2019) based on data from the QResearch and Clinical Practice Research Datalink (CPRD) databases. (2) Bioidentical progesterone was not included, but is known to be associated with no additional risk relative to estrogen alone. Footnotes: * = Statistically significant (p < 0.01). Sources: See template. | |||
Breast cancer
[edit]Drospirenone has been found to stimulate the proliferation and migration of breast cancer cells in preclinical research, similarly to certain other progestins.[64][65] However, some evidence suggests that drospirenone may do this more weakly than certain other progestins, like medroxyprogesterone acetate.[64][65] The combination of estradiol and drospirenone has been found to increase breast density, an established risk factor for breast cancer, in postmenopausal women.[66][67][68]
Data on risk of breast cancer in women with newer progestins like drospirenone are lacking at present.[69] Progestogen-only birth control is not generally associated with a higher risk of breast cancer.[69] Conversely, combined birth control and menopausal hormone therapy with an estrogen and a progestogen are associated with higher risks of breast cancer.[70][69][71]
Overdose
[edit]These have been no reports of serious adverse effects with overdose of drospirenone.[3] Symptoms that may occur in the event of an overdose may include nausea, vomiting, and vaginal bleeding.[3] There is no antidote for overdose of drospirenone and treatment of overdose should be based on symptoms.[3] Since drospirenone has antimineralocorticoid activity, levels of potassium and sodium should be measured and signs of metabolic acidosis should be monitored.[3]
Interactions
[edit]Inhibitors and inducers of the cytochrome P450 enzyme CYP3A4 may influence the levels and efficacy of drospirenone.[3] Treatment for 10 days with 200 mg twice daily ketoconazole, a strong CYP3A4 inhibitor among other actions, has been found to result in a moderate 2.0- to 2.7-fold increase in exposure to drospirenone.[3] Drospirenone does not appear to influence the metabolism of omeprazole (metabolized via CYP2C19), simvastatin (metabolized via CYP3A4), or midazolam (metabolized via CYP3A4), and likely does not influence the metabolism of other medications that are metabolized via these pathways.[3] Drospirenone may interact with potassium-sparing medications such as ACE inhibitors, angiotensin II receptor antagonists, potassium-sparing diuretics, potassium supplements, heparin, antimineralocorticoids, and nonsteroidal anti-inflammatory drugs to further increase potassium levels.[3] This may increase the risk of hyperkalemia (high potassium levels).[3]
Pharmacology
[edit]Pharmacodynamics
[edit]Drospirenone binds with high affinity to the progesterone receptor (PR) and mineralocorticoid receptor (MR), with lower affinity to the androgen receptor (AR), and with very low affinity to the glucocorticoid receptor (GR).[1][72][73][5] It is an agonist of the PR and an antagonist of the MR and AR, and hence is a progestogen, antimineralocorticoid, and antiandrogen.[1][72][5][65] Drospirenone has no estrogenic activity and no appreciable glucocorticoid or antiglucocorticoid activity.[1][72][5][65]
| Progestogen | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Drospirenone | 19–70 | 1–65 | 0–1 | 1–6 | 100–500 | 0 | 0 |
| Progesterone | 100 | 0–80 | 0–1 | 6–35 | 100–1000 | 0 | 0 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, and aldosterone for the MR. Sources:[4][1][5][74][65] | |||||||
Progestogenic activity
[edit]Drospirenone is an agonist of the PR, the biological target of progestogens like progesterone.[1][72] It has about 35% of the affinity of promegestone for the PR and about 19 to 70% of the affinity of progesterone for the PR.[1][4][65] Drospirenone has antigonadotropic and functional antiestrogenic effects as a result of PR activation.[1][72] The ovulation-inhibiting dosage of drospirenone is 2 to 3 mg/day.[75][76][1][77] Inhibition of ovulation occurred in about 90% of women at a dose of 0.5 to 2 mg/day and in 100% of women at a dose of 3 mg/day.[78] The total endometrial transformation dose of drospirenone is about 50 mg per cycle, whereas its daily dose is 2 mg for partial transformation and 4 to 6 mg for full transformation.[1][79][78] The medication acts as a contraceptive by activating the PR, which suppresses the secretion of luteinizing hormone, inhibits ovulation, and alters the cervical membrane and endometrium.[80][3]
Due to its antigonadotropic effects, drospirenone inhibits the secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and suppresses gonadal sex hormone production, including of estradiol, progesterone, and testosterone.[1][81][4] Drospirenone alone at 4 mg/day has been found to suppress estradiol levels in premenopausal women to about 40 to 80 pg/mL depending on the time of the cycle.[81] No studies of the antigonadotropic effects of drospirenone or its influence on hormone levels appear to have been conducted in men.[82][83][84] In male cynomolgus monkeys however, 4 mg/kg/day oral drospirenone strongly suppressed testosterone levels.[72]
Antimineralocorticoid activity
[edit]Drospirenone is an antagonist of the MR, the biological target of mineralocorticoids like aldosterone, and hence is an antimineralocorticoid.[72] It has about 100 to 500% of the affinity of aldosterone for the MR and about 50 to 230% of the affinity of progesterone for the MR.[1][4][74][65] Drospirenone is about 5.5 to 11 times more potent as an antimineralocorticoid than spironolactone in animals.[72][78][85] Accordingly, 3 to 4 mg drospirenone is said to be equivalent to about 20 to 25 mg spironolactone in terms of antimineralocorticoid activity.[86][3] It has been said that the pharmacological profile of drospirenone more closely resembles that of progesterone than other progestins due to its antimineralocorticoid activity.[72] Drospirenone is the only clinically used progestogen with prominent antimineralocorticoid activity besides progesterone.[1] For comparison to progesterone, a 200 mg dose of oral progesterone is considered to be approximately equivalent in antimineralocorticoid effect to a 25 to 50 mg dose of spironolactone.[87] Both drospirenone and progesterone are actually weak partial agonists of the MR in the absence of mineralocorticoids.[5][4][65]
Due to its antimineralocorticoid activity, drospirenone increases natriuresis, decreases water retention and blood pressure, and produces compensatory increases in plasma renin activity as well as circulating levels and urinary excretion of aldosterone.[4][88][1] This has been shown to occur at doses of 2 to 4 mg/day.[4] Similar effects occur during the luteal phase of the menstrual cycle due to increased progesterone levels and the resulting antagonism of the MR.[4] Estrogens, particularly ethinylestradiol, activate liver production of angiotensinogen and increase levels of angiotensinogen and angiotensin II, thereby activating the renin–angiotensin–aldosterone system.[4][1] As a result, they can produce undesirable side effects including increased sodium excretion, water retention, weight gain, and increased blood pressure.[4] Progesterone and drospirenone counteract these undesirable effects via their antimineralocorticoid activity.[4] Accumulating research indicates that antimineralocorticoids like drospirenone and spironolactone may also have positive effects on adipose tissue and metabolic health.[89][90]
Antiandrogenic activity
[edit]Drospirenone is an antagonist of the AR, the biological target of androgens like testosterone and dihydrotestosterone (DHT).[1][4] It has about 1 to 65% of the affinity of the synthetic anabolic steroid metribolone for the AR.[1][4][5][65] The medication is more potent as an antiandrogen than spironolactone, but is less potent than cyproterone acetate, with about 30% of its antiandrogenic activity in animals.[1][91][72][78] Progesterone displays antiandrogenic activity in some assays similarly to drospirenone,[4] although this issue is controversial and many researchers regard progesterone as having no significant antiandrogenic activity.[92][1][5]
Drospirenone shows antiandrogenic effects on the serum lipid profile, including higher HDL cholesterol and triglyceride levels and lower LDL cholesterol levels, at a dose of 3 mg/day in women.[4] The medication does not inhibit the effects of ethinylestradiol on sex hormone-binding globulin (SHBG) and serum lipids, in contrast to androgenic progestins like levonorgestrel but similarly to other antiandrogenic progestins like cyproterone acetate.[4][1][77] SHBG levels are significantly higher with ethinylestradiol and cyproterone acetate than with ethinylestradiol and drospirenone, owing to the more potent antiandrogenic activity of cyproterone acetate relative to drospirenone.[93] Androgenic progestins like levonorgestrel have been found to inhibit the procoagulatory effects of estrogens like ethinylestradiol on hepatic synthesis of coagulation factors, whereas this may occur less or not at all with weakly androgenic progestins like desogestrel and antiandrogenic progestins like drospirenone.[11][63][59][60][61][62]
Other activity
[edit]Drospirenone stimulates the proliferation of MCF-7 breast cancer cells in vitro, an action that is independent of the classical PRs and is instead mediated via the progesterone receptor membrane component-1 (PGRMC1).[94] Certain other progestins act similarly in this assay, whereas progesterone acts neutrally.[94] It is unclear if these findings may explain the different risks of breast cancer observed with progesterone and progestins in clinical studies.[69]
Pharmacokinetics
[edit]Absorption
[edit]The oral bioavailability of drospirenone is between 66 and 85%.[1][4][5] Peak levels occur 1 to 6 hours after an oral dose.[1][4][3][85] Levels are about 27 ng/mL after a single 4 mg dose.[3] There is 1.5- to 2-fold accumulation in drospirenone levels with continuous administration, with steady-state levels of drospirenone achieved after 7 to 10 days of administration.[1][3][4] Peak levels of drospirenone at steady state with 4 mg/day drospirenone are about 41 ng/mL.[3] With the combination of 30 μg/day ethinylestradiol and 3 mg/day drospirenone, peak levels of drospirenone after a single dose are 35 ng/mL, and levels at steady state are 60 to 87 ng/mL at peak and 20 to 25 ng/mL at trough.[4][1] The pharmacokinetics of oral drospirenone are linear with a single dose across a dose range of 1 to 10 mg.[3][4] Intake of drospirenone with food does not influence the absorption of drospirenone.[3]
Distribution
[edit]The distribution half-life of drospirenone is about 1.6 to 2 hours.[4][1] The apparent volume of distribution of drospirenone is approximately 4 L/kg.[3] The plasma protein binding of drospirenone is 95 to 97%.[3][1] It is bound to albumin and 3 to 5% circulates freely or unbound.[3][1] Drospirenone has no affinity for sex hormone-binding globulin (SHBG) or corticosteroid-binding globulin (CBG), and hence is not bound by these plasma proteins in the circulation.[1]
Metabolism
[edit]The metabolism of drospirenone is extensive.[4] It is metabolized into the acid form of drospirenone by opening of its lactone ring.[1][3] The medication is also metabolized by reduction of its double bond between the C4 and C5 positions and subsequent sulfation.[1][3] The two major metabolites of drospirenone are drospirenone acid and 4,5-dihydrodrospirenone 3-sulfate, and are both formed independently of the cytochrome P450 system.[3][4] Neither of these metabolites are known to be pharmacologically active.[3] Drospirenone also undergoes oxidative metabolism by CYP3A4.[3][4][7][8]
Elimination
[edit]Drospirenone is excreted in urine and feces, with slightly more excreted in feces than in urine.[3] Only trace amounts of unchanged drospirenone can be found in urine and feces.[3] At least 20 different metabolites can be identified in urine and feces.[4] Drospirenone and its metabolites are excreted in urine about 38% as glucuronide conjugates, 47% as sulfate conjugates, and less than 10% in unconjugated form.[4] In feces, excretion is about 17% glucuronide conjugates, 20% sulfate conjugates, and 33% unconjugated.[4]
The elimination half-life of drospirenone is between 25 and 33 hours.[3][4][1] The half-life of drospirenone is unchanged with repeated administration.[3] Elimination of drospirenone is virtually complete 10 days after the last dose.[4][3]
Chemistry
[edit] Chemical structures of spirolactones
|
Drospirenone, also known as 1,2-dihydrospirorenone or as 17β-hydroxy-6β,7β:15β,16β-dimethylene-3-oxo-17α-pregn-4-ene-21-carboxylic acid, γ-lactone, is a synthetic steroidal 17α-spirolactone, or more simply a spirolactone.[9][95] It is an analogue of other spirolactones like spironolactone, canrenone, and spirorenone.[9][95] Drospirenone differs structurally from spironolactone only in that the C7α acetyl thio substitution of spironolactone has been removed and two methylene groups have been substituted in at the C6β–7β and C15β–16β positions.[96]
Spirolactones like drospirenone and spironolactone are derivatives of progesterone, which likewise has progestogenic and antimineralocorticoid activity.[97][98][99] The loss of the C7α acetylthio group of spironolactone, a compound with negligible progestogenic activity,[100][101] appears to be involved in the restoration of progestogenic activity in drospirenone, as SC-5233, the analogue of spironolactone without a C7α substitution, has potent progestogenic activity similarly to drospirenone.[102]
History
[edit]Drospirenone was patented in 1976 and introduced for medical use in 2000.[14][15] Schering AG of Germany has been granted several patents on the production of drospirenone, including WIPO and US patents, granted in 1998 and 2000, respectively.[103][104] It was introduced for medical use in combination with ethinylestradiol as a combined birth control pill in 2000.[14] Drospirenone is sometimes described as a "fourth-generation" progestin based on its time of introduction.[16][17] The medication was approved for use in menopausal hormone therapy in combination with estradiol in 2005.[23] Drospirenone was introduced for use as a progestogen-only birth control pill in 2019.[3] A combined birth control pill containing estetrol and drospirenone was approved in 2021.[105]
Society and culture
[edit]Generic names
[edit]Drospirenone is the generic name of the drug and its INN, USAN, BAN, and JAN, while drospirénone is its DCF.[9] Its name is a shortened form of the name 1,2-dihydrospirorenone or dihydrospirenone.[9][95] Drospirenone is also known by its developmental code names SH-470 and ZK-30595 (alone), BAY 86-5300, BAY 98-7071, and SH-T-00186D (in combination with ethinylestradiol), BAY 86-4891 (in combination with estradiol), and FSN-013 (in combination with estetrol).[9][95][106][107][108][109][105]
Brand names
[edit]Drospirenone is marketed in combination with an estrogen under a variety of brand names throughout the world.[9] Among others, it is marketed in combination with ethinylestradiol under the brand names Yasmin and Yaz, in combination with estetrol under the brand name Nextstellis, and in combination with estradiol under the brand name Angeliq.[9][105]
Availability
[edit]Drospirenone is marketed widely throughout the world.[9]
Generation
[edit]Drospirenone has been categorized as a "fourth-generation" progestin.[65]
Litigation
[edit]Many lawsuits have been filed against Bayer, the manufacturer of drospirenone, due to the higher risk of venous thromboembolism (VTE) that has been observed with combined birth control pills containing drospirenone and certain other progestins relative to the risk with levonorgestrel-containing combined birth control pills.[55]
In July 2012, Bayer notified its stockholders that there were more than 12,000 such lawsuits against the company involving Yaz, Yasmin, and other birth control pills with drospirenone.[110] They also noted that the company by then had settled 1,977 cases for US$402.6 million, for an average of US$212,000 per case, while setting aside US$610.5 million to settle the others.[110]
As of 17 July 2015, there have been at least 4,000 lawsuits and claims still pending regarding VTE related to drospirenone.[111] This is in addition to around 10,000 claims that Bayer has already settled without admitting liability.[111] These claims of VTE have amounted to US$1.97 billion.[111] Bayer also reached a settlement for arterial thromboembolic events, including stroke and heart attacks, for US$56.9 million.[111]
Research
[edit]A combination of ethinylestradiol, drospirenone, and prasterone is under development by Pantarhei Bioscience as a combined birth control pill for prevention of pregnancy in women.[112] It includes prasterone (dehydroepiandrosterone; DHEA), an oral androgen prohormone, to replace testosterone and avoid testosterone deficiency caused by suppression of testosterone by ethinylestradiol and drospirenone.[112] As of August 2018, the formulation is in phase II/III clinical trials.[112]
Drospirenone has been suggested for potential use as a progestin in male hormonal contraception.[82]
Drospirenone has been studied in forms for parenteral administration.[113][114][115][116]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1 (sup1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au Slynd-drospirenone tablet, film coated drug label/data at DailyMed from U.S. National Library of Medicine, National Institutes of Health.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah Krattenmacher R (July 2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- ^ a b c d e f g h i Stanczyk FZ, Hapgood JP, Winer S, Mishell DR (April 2013). "Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects". Endocrine Reviews. 34 (2): 171–208. doi:10.1210/er.2012-1008. PMC 3610676. PMID 23238854.
- ^ Gaspard U, Endrikat J, Desager JP, Buicu C, Gerlinger C, Heithecker R (April 2004). "A randomized study on the influence of oral contraceptives containing ethinylestradiol combined with drospirenone or desogestrel on lipid and lipoprotein metabolism over a period of 13 cycles". Contraception. 69 (4): 271–278. doi:10.1016/j.contraception.2003.11.003. PMID 15033400.
- ^ a b Bachmann G, Kopacz S (November 2009). "Drospirenone/ethinyl estradiol 3 mg/20 mug (24/4 day regimen): hormonal contraceptive choices - use of a fourth-generation progestin". Patient Preference and Adherence. 3: 259–264. doi:10.2147/PPA.S3901. PMC 2778416. PMID 19936169.
- ^ a b Wiesinger H, Berse M, Klein S, Gschwend S, Höchel J, Zollmann FS, et al. (December 2015). "Pharmacokinetic interaction between the CYP3A4 inhibitor ketoconazole and the hormone drospirenone in combination with ethinylestradiol or estradiol". British Journal of Clinical Pharmacology. 80 (6): 1399–1410. doi:10.1111/bcp.12745. PMC 4693482. PMID 26271371.
- ^ a b c d e f g h i j k drospirenone at Drugs.com: Multum Consumer Information
- ^ Krattenmacher R (July 2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- ^ a b c d e f g h i j Han L, Jensen JT (December 2015). "Does the Progestogen Used in Combined Hormonal Contraception Affect Venous Thrombosis Risk?". Obstetrics and Gynecology Clinics of North America. 42 (4). Elsevier: 683–698. doi:10.1016/j.ogc.2015.07.007. eISSN 1558-0474. PMID 26598309.
- ^ Oelkers W (December 2000). "Drospirenone--a new progestogen with antimineralocorticoid activity, resembling natural progesterone". The European Journal of Contraception & Reproductive Health Care (Review). 5 (Suppl 3): 17–24. doi:10.1080/14730782.2000.12288986. PMID 11246598. S2CID 35051390.
- ^ Oelkers W (December 2002). "Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone". The European Journal of Contraception & Reproductive Health Care (Review). 7 (Suppl 3): 19–26, discussion 42–3. doi:10.1080/14730782.2000.12288986. PMID 12659403.
- ^ a b c Ravina E (11 January 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. pp. 193–. ISBN 978-3-527-32669-3.
- ^ a b Alapi EM, Fischer J (2006). "Part III: Table of Selected Analogue Classes". In Fischer J, Ganellin CR (eds.). Analogue-based Drug Discovery. John Wiley & Sons. p. 459. ISBN 978-3-527-60749-5.
- ^ a b Nelson AL (2007). "Combined Oral Contraceptives". In Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D (eds.). Contraceptive Technology (19th ed.). Ardent Media. p. 196. ISBN 978-1-59708-001-9.
- ^ a b Harper JC (20 April 2016). "Use of Oral Contraceptives for Management of Acne Vulgaris". In Del Rosso JQ, Zeichner JA (eds.). Advances in Acne Management, An Issue of Dermatologic Clinics, E-Book. Elsevier Health Sciences. p. 160. ISBN 978-0-323-41753-2.
- ^ Generic Yasmin Availability via yasminat Drugs.com
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Drospirenone; Ethinyl Estradiol - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ a b "Yaz- drospirenone and ethinyl estradiol kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ Cerner Multum, Inc. (11 June 2012). "drospirenone and ethinyl estradiol". Auckland, New Zealand: Drugs.com. Retrieved 24 October 2011.
- ^ a b c "Angeliq- drospirenone and estradiol tablet, film coated". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ Maclennan AH, Broadbent JL, Lester S, Moore V (October 2004). "Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes". The Cochrane Database of Systematic Reviews. 2004 (4): CD002978. doi:10.1002/14651858.CD002978.pub2. PMC 7004247. PMID 15495039.
- ^ Torgerson DJ, Bell-Syer SE (June 2001). "Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials". JAMA. 285 (22): 2891–2897. doi:10.1001/jama.285.22.2891. PMID 11401611. S2CID 25078579.
- ^ a b Whitehead M (March 2006). "Hormone replacement therapy with estradiol and drospirenone: an overview of the clinical data". The Journal of the British Menopause Society. 12 (Suppl 1): 4–7. doi:10.1258/136218006775992185. PMID 16513012. S2CID 38095916.
- ^ Majumder A, Sanyal D (2017). "Outcome and preferences in male-to-female subjects with gender dysphoria: Experience from Eastern India". Indian Journal of Endocrinology and Metabolism. 21 (1): 21–25. doi:10.4103/2230-8210.196000. PMC 5240066. PMID 28217493.
- ^ Majumder A, Chatterjee S, Maji D, Roychaudhuri S, Ghosh S, Selvan C, et al. (2020). "IDEA Group Consensus Statement on Medical Management of Adult Gender Incongruent Individuals Seeking Gender Reaffirmation as Female". Indian Journal of Endocrinology and Metabolism. 24 (2): 128–135. doi:10.4103/ijem.IJEM_593_19. PMC 7333765. PMID 32699777.
- ^ Lanza di Scalea T, Pearlstein T (June 2017). "Premenstrual Dysphoric Disorder". The Psychiatric Clinics of North America. 40 (2): 201–216. doi:10.1016/j.psc.2017.01.002. PMID 28477648.
- ^ Ma S, Song SJ (June 2023). "Oral contraceptives containing drospirenone for premenstrual syndrome". The Cochrane Database of Systematic Reviews. 2023 (6): CD006586. doi:10.1002/14651858.CD006586.pub5. PMC 10289136. PMID 37365881.
- ^ Christiansen C (October 2005). "Effects of drospirenone/estrogen combinations on bone metabolism". Climacteric. 8 (Suppl 3): 35–41. doi:10.1080/13697130500330283. PMID 16203654. S2CID 42803561.
- ^ a b Archer DF (February 2007). "Drospirenone and estradiol: a new option for the postmenopausal woman". Climacteric. 10 (Suppl 1): 3–10. doi:10.1080/13697130601114859. PMID 17364592. S2CID 9221524.
- ^ a b "Drospirenone in HRT?". Drug and Therapeutics Bulletin. 47 (4): 41–44. April 2009. doi:10.1136/dtb.2009.03.0011. PMID 19357298. S2CID 1909717.
- ^ Foidart JM, Faustmann T (December 2007). "Advances in hormone replacement therapy: weight benefits of drospirenone, a 17alpha-spirolactone-derived progestogen". Gynecological Endocrinology. 23 (12): 692–699. doi:10.1080/09513590701582323. PMID 18075844. S2CID 12572825.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 23 December 2019.
- ^ Center for Drug Evaluation and Research. "Drug Safety and Availability - FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone". www.fda.gov. Retrieved 7 November 2017.
- ^ "NEXTSTELLIS (drospirenone and estetrol tablets)" (PDF). Mayne Pharma. U.S. Food and Drug Administration. April 2021.
- ^ "U.S. FDA Approved NEXTSTELLIS®, New Oral Contraceptive" (PDF). Mayne Pharma. Archived from the original (PDF) on 17 April 2021.
- ^ "Mayne Pharma and Mithra Announce FDA Approval of New Oral Contraceptive Nextstellis®" (PDF).
- ^ "Ocella- drospirenone and ethinyl estradiol kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ "Syeda- drospirenone and ethinyl estradiol kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ "Yasmin- drospirenone and ethinyl estradiol kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ "Zarah- drospirenone and ethinyl estradiol kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ "Beyaz- drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ "Tydemy- drospirenone, ethinyl estradiol and levomefolate calcium and levomefolate calcium kit". DailyMed. U.S. National Library of Medicine. Retrieved 17 April 2021.
- ^ "Estetrol monohydrate and Drospirenone Tablets" (PDF). Searchlight Pharma Inc. Archived from the original (PDF) on 13 April 2021. Retrieved 15 January 2022.
- ^ "Mithra and Searchlight Pharma Announce Nextstellis Approval in Canada". Searchlight Pharma (Press release). 8 March 2021. Archived from the original on 19 April 2021. Retrieved 17 April 2021.
- ^ a b c Bayer (25 March 2013). "Summary of Product Characteristics (SPC): Yasmin". London: electronic Medicines Compendium (eMC), Datapharm. Retrieved 24 April 2014.
4.3. Contraindications: • Severe chronic kidney disease or acute kidney failure. • Presence or history of severe hepatic disease as long as liver function values have not returned to normal.
- ^ a b Bayer (10 April 2012). "Yasmin full prescribing information" (PDF). Silver Spring, Md.: Food and Drug Administration (FDA). Retrieved 14 April 2012.
4. Contraindications: • Renal impairment. • Adrenal insufficiency. • Liver disease.
- ^ Nelson AL, Cwiak C (2011). "Combined oral contraceptives (COCs)". In Hatcher RA, Trussell J, Nelson AL, Cates Jr W, Kowal D, Policar MS (eds.). Contraceptive Technology (20th revised ed.). New York: Ardent Media. pp. 249–341. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734.
- ^ a b Heit JA, Spencer FA, White RH (January 2016). "The epidemiology of venous thromboembolism". Journal of Thrombosis and Thrombolysis. 41 (1): 3–14. doi:10.1007/s11239-015-1311-6. PMC 4715842. PMID 26780736.
- ^ a b c d e Bateson D, Butcher BE, Donovan C, Farrell L, Kovacs G, Mezzini T, et al. (2016). "Risk of venous thromboembolism in women taking the combined oral contraceptive: A systematic review and meta-analysis". Australian Family Physician. 45 (1): 59–64. PMID 27051991.
- ^ a b "FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone". Food and Drug Administration. 27 April 2019. Archived from the original on 27 April 2019.
- ^ Vinogradova Y, Coupland C, Hippisley-Cox J (January 2019). "Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases". BMJ. 364: k4810. doi:10.1136/bmj.k4810. PMC 6326068. PMID 30626577.
- ^ a b c Batur P, Casey PM (February 2017). "Drospirenone Litigation: Does the Punishment Fit the Crime?". Journal of Women's Health. 26 (2): 99–102. doi:10.1089/jwh.2016.6092. PMID 27854556.
- ^ a b Sitruk-Ware R (November 2016). "Hormonal contraception and thrombosis". Fertility and Sterility. 106 (6): 1289–1294. doi:10.1016/j.fertnstert.2016.08.039. PMID 27678035.
- ^ Oedingen C, Scholz S, Razum O (May 2018). "Systematic review and meta-analysis of the association of combined oral contraceptives on the risk of venous thromboembolism: The role of the progestogen type and estrogen dose". Thrombosis Research. 165: 68–78. doi:10.1016/j.thromres.2018.03.005. PMID 29573722.
- ^ Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME (June 2018). "A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception". International Journal of Gynaecology and Obstetrics. 141 (3): 287–294. doi:10.1002/ijgo.12455. PMC 5969307. PMID 29388678.
- ^ a b Wiegratz I, Kuhl H (September 2006). "Metabolic and clinical effects of progestogens". The European Journal of Contraception & Reproductive Health Care. 11 (3): 153–161. doi:10.1080/13625180600772741. PMID 17056444. S2CID 27088428.
- ^ a b Kuhl H (May 1996). "Effects of progestogens on haemostasis". Maturitas. 24 (1–2): 1–19. doi:10.1016/0378-5122(96)00994-2. PMID 8794429.
- ^ a b Sitruk-Ware R, Nath A (February 2013). "Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills". Best Practice & Research. Clinical Endocrinology & Metabolism. 27 (1): 13–24. doi:10.1016/j.beem.2012.09.004. PMID 23384742.
- ^ a b Nelson AL (2015). "An update on new orally administered contraceptives for women". Expert Opinion on Pharmacotherapy. 16 (18): 2759–2772. doi:10.1517/14656566.2015.1100173. PMID 26512437. S2CID 207481206.
- ^ a b c Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G (October 2017). "Pharmacodynamics of combined estrogen-progestin oral contraceptives: 2. effects on hemostasis". Expert Review of Clinical Pharmacology. 10 (10): 1129–1144. doi:10.1080/17512433.2017.1356718. PMID 28712325. S2CID 205931204.
- ^ a b Simoncini T, Genazzani AR (February 2010). "A review of the cardiovascular and breast actions of drospirenone in preclinical studies". Climacteric. 13 (1): 22–33. doi:10.3109/13697130903437375. PMID 19938948. S2CID 4306359.
- ^ a b c d e f g h i j Africander D, Verhoog N, Hapgood JP (June 2011). "Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception". Steroids. 76 (7): 636–652. doi:10.1016/j.steroids.2011.03.001. PMID 21414337. S2CID 23630452.
- ^ Palacios S, Mejía A (November 2016). "Progestogen safety and tolerance in hormonal replacement therapy". Expert Opinion on Drug Safety. 15 (11): 1515–1525. doi:10.1080/14740338.2016.1223041. PMID 27548404. S2CID 31497860.
- ^ Caglayan EK, Caglayan K, Alkis I, Arslan E, Okur A, Banli O, et al. (August 2015). "Factors Associated with Mammographic Density in Postmenopausal Women". Journal of Menopausal Medicine. 21 (2): 82–88. doi:10.6118/jmm.2015.21.2.82. PMC 4561745. PMID 26357645.
- ^ Hirschberg AL, Tani E, Brismar K, Lundström E (August 2019). "Effects of drospirenone and norethisterone acetate combined with estradiol on mammographic density and proliferation of breast epithelial cells-A prospective randomized trial". Maturitas. 126: 18–24. doi:10.1016/j.maturitas.2019.04.205. PMID 31239112.
- ^ a b c d Trabert B, Sherman ME, Kannan N, Stanczyk FZ (April 2020). "Progesterone and Breast Cancer". Endocrine Reviews. 41 (2): 320–344. doi:10.1210/endrev/bnz001. PMC 7156851. PMID 31512725.
- ^ Collaborative Group on Hormonal Factors in Breast Cancer, et al. (Collaborative Group on Hormonal Factors in Breast Cancer) (September 2019). "Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence". Lancet. 394 (10204): 1159–1168. doi:10.1016/S0140-6736(19)31709-X. PMC 6891893. PMID 31474332.
- ^ Sturdee DW (August 2013). "Are progestins really necessary as part of a combined HRT regimen?". Climacteric. 16 (Suppl 1): 79–84. doi:10.3109/13697137.2013.803311. PMID 23651281. S2CID 21894200.
- ^ a b c d e f g h i j Muhn P, Fuhrmann U, Fritzemeier KH, Krattenmacher R, Schillinger E (June 1995). "Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity". Annals of the New York Academy of Sciences. 761 (3): 311–335. Bibcode:1995NYASA.761..311M. doi:10.1111/j.1749-6632.1995.tb31386.x. PMID 7625729. S2CID 36861309.
- ^ Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH (October 1996). "The novel progestin drospirenone and its natural counterpart progesterone: biochemical profile and antiandrogenic potential". Contraception. 54 (4): 243–251. doi:10.1016/S0010-7824(96)00195-3. PMID 8922878.
- ^ a b Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM (July 2014). "Potency of progestogens used in hormonal therapy: toward understanding differential actions". The Journal of Steroid Biochemistry and Molecular Biology. 142: 39–47. doi:10.1016/j.jsbmb.2013.08.001. PMID 23954501. S2CID 12142015.
- ^ Bastianelli C, Farris M, Rosato E, Brosens I, Benagiano G (November 2018). "Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation". Expert Review of Clinical Pharmacology. 11 (11): 1085–1098. doi:10.1080/17512433.2018.1536544. PMID 30325245. S2CID 53246678.
- ^ Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–557. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ^ a b Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
- ^ a b c d Elger W, Beier S, Pollow K, Garfield R, Shi SQ, Hillisch A (November 2003). "Conception and pharmacodynamic profile of drospirenone". Steroids. 68 (10–13): 891–905. doi:10.1016/j.steroids.2003.08.008. PMID 14667981. S2CID 41756726.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 (Suppl 1): S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ "Drospirenone". pubchem.ncbi.nlm.nih.gov.
- ^ a b Hadji P, Colli E, Regidor PA (December 2019). "Bone health in estrogen-free contraception". Osteoporosis International. 30 (12): 2391–2400. doi:10.1007/s00198-019-05103-6. PMC 7203087. PMID 31446440.
- ^ a b Cornia PB, Anawalt BD (2005). "Male hormonal contraceptives: a potentially patentable and profitable product". Expert Opinion on Therapeutic Patents. 15 (12): 1727–1737. doi:10.1517/13543776.15.12.1727. ISSN 1354-3776. S2CID 83941717.
- ^ Nieschlag E (November 2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–470. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
- ^ Nieschlag E, Behre HM (2012). "The essential role of testosterone in hormonal male contraception". In Nieschlag E, Behre HM, Nieschlag S (eds.). Testosterone. Cambridge University Press. pp. 470–493. doi:10.1017/CBO9781139003353.023. ISBN 978-1-139-00335-3.
- ^ a b Stanczyk FZ (2007). "Structure –Function Relationships, Pharmacokinetics, and Potency of Orally and Parenterally Administered Progestogens". Treatment of the Postmenopausal Woman. Academic Press. pp. 779–798. doi:10.1016/B978-012369443-0/50067-3. ISBN 978-0-12-369443-0.
- ^ Schneider HP, Naftolin F (22 September 2004). Climacteric Medicine - Where Do We Go?: Proceedings of the 4th Workshop of the International Menopause Society. CRC Press. pp. 133–. ISBN 978-0-203-02496-6.
- ^ Simon JA (December 1995). "Micronized progesterone: vaginal and oral uses". Clinical Obstetrics and Gynecology. 38 (4): 902–914. doi:10.1097/00003081-199538040-00024. PMID 8616985.
- ^ Genazzani AR, Mannella P, Simoncini T (February 2007). "Drospirenone and its antialdosterone properties". Climacteric. 10 Suppl 1 (Supplement 1): 11–18. doi:10.1080/13697130601114891. PMID 17364593. S2CID 24872884.
- ^ Infante M, Armani A, Marzolla V, Fabbri A, Caprio M (2019). "Adipocyte Mineralocorticoid Receptor". Vitamins and Hormones. 109. Elsevier: 189–209. doi:10.1016/bs.vh.2018.10.005. ISBN 978-0-12-817782-2. PMID 30678856. S2CID 59251387.
- ^ Giordano A, Frontini A, Cinti S (June 2016). "Convertible visceral fat as a therapeutic target to curb obesity". Nature Reviews. Drug Discovery. 15 (6): 405–424. doi:10.1038/nrd.2016.31. hdl:11566/235770. PMID 26965204. S2CID 2632187.
- ^ Sitruk-Ware R, Husmann F, Thijssen JH, Skouby SO, Fruzzetti F, Hanker J, et al. (September 2004). "Role of progestins with partial antiandrogenic effects". Climacteric. 7 (3): 238–254. doi:10.1080/13697130400001307. PMID 15669548. S2CID 23112620.
- ^ Yeh YT, Chang CW, Wei RJ, Wang SN (2013). "Progesterone and related compounds in hepatocellular carcinoma: basic and clinical aspects". BioMed Research International. 2013: 290575. doi:10.1155/2013/290575. PMC 3581253. PMID 23484104.
- ^ Schindler AE (2015). "Hormonal Contraceptives: Progestogen and Thrombotic Risk". Frontiers in Gynecological Endocrinology. ISGE Series. Springer. pp. 69–75. doi:10.1007/978-3-319-09662-9_8. ISBN 978-3-319-09661-2. ISSN 2197-8735.
- ^ a b Neubauer H, Ma Q, Zhou J, Yu Q, Ruan X, Seeger H, et al. (October 2013). "Possible role of PGRMC1 in breast cancer development". Climacteric. 16 (5): 509–513. doi:10.3109/13697137.2013.800038. PMID 23758160. S2CID 29808177.
- ^ a b c d Negwer M, Scharnow HG (4 October 2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 2539. ISBN 978-3-527-30247-5.
- ^ Carp HJ (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. pp. 115–. ISBN 978-3-319-14385-9.
- ^ Ménard J (March 2004). "The 45-year story of the development of an anti-aldosterone more specific than spironolactone". Molecular and Cellular Endocrinology. 217 (1–2): 45–52. doi:10.1016/j.mce.2003.10.008. PMID 15134800. S2CID 19701784.
[Spironolactone] was synthesized after the demonstration of the natriuretic effect of progesterone (Landau et al., 1955).
- ^ Jameson JL, De Groot LJ (18 May 2010). Endocrinology - E-Book: Adult and Pediatric. Elsevier Health Sciences. pp. 2401–. ISBN 978-1-4557-1126-0.
[Spironolactone] is a potent antimineralocorticoid which was developed as a progestational analog [...]
- ^ Aldosterone. Elsevier Science. 23 January 2019. p. 46. ISBN 978-0-12-817783-9.
In addition to spironolactone, which is a derivative of progesterone [...]
- ^ Hu X, Li S, McMahon EG, Lala DS, Rudolph AE (August 2005). "Molecular mechanisms of mineralocorticoid receptor antagonism by eplerenone". Mini Reviews in Medicinal Chemistry. 5 (8): 709–718. doi:10.2174/1389557054553811. PMID 16101407.
- ^ Nakajima ST, Brumsted JR, Riddick DH, Gibson M (July 1989). "Absence of progestational activity of oral spironolactone". Fertility and Sterility. 52 (1): 155–158. doi:10.1016/s0015-0282(16)60807-5. PMID 2744183.
- ^ Hertz R, Tullner WW (November 1958). "Progestational activity of certain steroid-17-spirolactones". Proceedings of the Society for Experimental Biology and Medicine. 99 (2): 451–452. doi:10.3181/00379727-99-24380. PMID 13601900. S2CID 20150966.
- ^ WO patent 9806738, Mohr, Jörg-Thorsten & Nickisch, Klaus, "PROCESS FOR PRODUCING DROSPIRENONE (6ss,7ss;15ss,16ss-DIMETHYLENE-3-OXO-17 alpha -PREGN-4-EN-21,17-CARBOLACTONE, DRSP), AS WELL AS 7 alpha -(3-HYDROXY-1-PROPYL)-6ss,7ss;15ss,16ss-DIMETHYLENE-5ss-ANDROSTANE-3ss,5,17ss-TRIOL (ZK 92836) AND 6ss,7ss;15ss,16ss-DIMETHYLENE-5ss HYDROXY-5-OXO-17 alpha -ANDROSTANE-21, 17-CARBOLACTONE", issued 1998-02-19, assigned to Shering AG
- ^ US patent 6121465, Mohr, Joerg-Thorston & Nickisch, Klaus, "Process for production drospirenone and intermediate products of the process", issued 2000-09-19, assigned to Scheiring AG and Bayer Schering Pharma
- ^ a b c "Drospirenone/Estetrol - Mithra Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG.
- ^ "Ethinylestradiol/drospirenone". AdisInsight. Springer Nature Switzerland AG.
- ^ "Ethinylestradiol/drospirenone/folic acid". AdisInsight. Springer Nature Switzerland AG.
- ^ "Drospirenone/ethinylestradiol low-dose - Bayer HealthCare Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG.
- ^ "Estradiol/drospirenone - Bayer HealthCare Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG.
- ^ a b Feeley J, Kresge N (31 July 2012). "Bayer's Yasmin lawsuit settlements rise to $402.6 million". Bloomberg News. New York. Retrieved 11 November 2012.
- ^ a b c d "Quarterly Reports of Bayer". Bayer AG.
- ^ a b c "Drospirenone/estradiol/prasterone - ANI Pharmaceuticals/Pantarhei Bioscience". AdisInsight. Springer Nature Switzerland AG.
- ^ Nippe S, General S (September 2011). "Parenteral oil-based drospirenone microcrystal suspensions-evaluation of physicochemical stability and influence of stabilising agents". International Journal of Pharmaceutics. 416 (1): 181–188. doi:10.1016/j.ijpharm.2011.06.036. PMID 21729745.
- ^ Nippe S, General S (November 2012). "Combination of injectable ethinyl estradiol and drospirenone drug-delivery systems and characterization of their in vitro release". European Journal of Pharmaceutical Sciences. 47 (4): 790–800. doi:10.1016/j.ejps.2012.08.009. PMID 22940138.
- ^ Nippe S, Preuße C, General S (February 2013). "Evaluation of the in vitro release and pharmacokinetics of parenteral injectable formulations for steroids". European Journal of Pharmaceutics and Biopharmaceutics. 83 (2): 253–265. doi:10.1016/j.ejpb.2012.09.006. PMID 23116659.
- ^ Nippe S, General S (April 2015). "Investigation of injectable drospirenone organogels with regard to their rheology and comparison to non-stabilized oil-based drospirenone suspensions". Drug Development and Industrial Pharmacy. 41 (4): 681–691. doi:10.3109/03639045.2014.895375. PMID 24621345. S2CID 42932558.
Further reading
[edit]- Archer DF (February 2007). "Drospirenone and estradiol: a new option for the postmenopausal woman". Climacteric. 10 (Suppl 1): 3–10. doi:10.1080/13697130601114859. PMID 17364592. S2CID 9221524.
- Archer DF (February 2007). "Drospirenone-containing hormone therapy for postmenopausal women. Perspective on current data". The Journal of Reproductive Medicine. 52 (2 Suppl): 159–164. PMID 17477110.
- Archer DF (2007). "Drospirenone, a progestin with added value for hypertensive postmenopausal women". Menopause. 14 (3 Pt 1): 352–354. doi:10.1097/gme.0b013e31804d440b. PMID 17414576.
- Batur P, Casey PM (February 2017). "Drospirenone Litigation: Does the Punishment Fit the Crime?". Journal of Women's Health. 26 (2): 99–102. doi:10.1089/jwh.2016.6092. PMID 27854556.
- Bitzer J, Paoletti AM (2009). "Added benefits and user satisfaction with a low-dose oral contraceptive containing drospirenone: results of three multicentre trials". Clinical Drug Investigation. 29 (2): 73–78. doi:10.2165/0044011-200929020-00001. PMID 19133702. S2CID 10356578.
- Carranza-Lira S (2009). "Safety, efficacy and patient acceptability of drospirenone and estradiol in the treatment of menopausal vasomotor symptoms: a review". Clinical Interventions in Aging. 4: 59–62. doi:10.2147/CIA.S4117. PMC 2685225. PMID 19503766.
- Christiansen C (October 2005). "Effects of drospirenone/estrogen combinations on bone metabolism". Climacteric. 8 (Suppl 3): 35–41. doi:10.1080/13697130500330283. PMID 16203654. S2CID 42803561.
- Dickerson V (November 2002). "Quality of life issues. Potential role for an oral contraceptive containing ethinyl estradiol and drospirenone". The Journal of Reproductive Medicine. 47 (11 Suppl): 985–993. PMID 12497673.
- Fenton C, Wellington K, Moen MD, Robinson DM (2007). "Drospirenone/ethinylestradiol 3mg/20microg (24/4 day regimen): a review of its use in contraception, premenstrual dysphoric disorder and moderate acne vulgaris". Drugs. 67 (12): 1749–1765. doi:10.2165/00003495-200767120-00007. PMID 17683173. S2CID 46976925.
- Foidart JM (October 2005). "Added benefits of drospirenone for compliance". Climacteric. 8 (Suppl 3): 28–34. doi:10.1080/13697130500330309. PMID 16203653. S2CID 31883491.
- Foidart JM, Faustmann T (December 2007). "Advances in hormone replacement therapy: weight benefits of drospirenone, a 17alpha-spirolactone-derived progestogen". Gynecological Endocrinology. 23 (12): 692–699. doi:10.1080/09513590701582323. PMID 18075844. S2CID 12572825.
- Genazzani AR, Mannella P, Simoncini T (February 2007). "Drospirenone and its antialdosterone properties". Climacteric. 10 (Suppl 1): 11–18. doi:10.1080/13697130601114891. PMID 17364593. S2CID 24872884.
- Han L, Jensen JT (October 2014). "Expert opinion on a flexible extended regimen of drospirenone/ethinyl estradiol contraceptive". Expert Opinion on Pharmacotherapy. 15 (14): 2071–2079. doi:10.1517/14656566.2014.949237. PMID 25186109. S2CID 25338932.
- Heinemann LA, Dinger J (2004). "Safety of a new oral contraceptive containing drospirenone". Drug Safety. 27 (13): 1001–1018. doi:10.2165/00002018-200427130-00003. PMID 15471507. S2CID 1773936.
- Idota N, Kobayashi M, Miyamori D, Kakiuchi Y, Ikegaya H (March 2015). "Drospirenone detected in postmortem blood of a young woman with pulmonary thromboembolism: A case report and review of the literature". Legal Medicine. 17 (2): 109–115. doi:10.1016/j.legalmed.2014.10.001. PMID 25454533.
- Keam SJ, Wagstaff AJ (2003). "Ethinylestradiol/drospirenone: a review of its use as an oral contraceptive". Treatments in Endocrinology. 2 (1): 49–70. doi:10.2165/00024677-200302010-00005. PMID 15871554. S2CID 209144694.
- Krattenmacher R (July 2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. doi:10.1016/S0010-7824(00)00133-5. PMID 11024226.
- Larivée N, Suissa S, Khosrow-Khavar F, Tagalakis V, Filion KB (September 2017). "Drospirenone-containing oral contraceptive pills and the risk of venous thromboembolism: a systematic review of observational studies". BJOG. 124 (10): 1490–1499. doi:10.1111/1471-0528.14623. PMID 28276140.
- Lete I, Chabbert-Buffet N, Jamin C, Lello S, Lobo P, Nappi RE, et al. (2015). "Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: A literature review". The European Journal of Contraception & Reproductive Health Care. 20 (5): 329–343. doi:10.3109/13625187.2015.1050091. PMID 26007631. S2CID 41601833.
- Li J, Ren J, Sun W (March 2017). "A comparative systematic review of Yasmin (drospirenone pill) versus standard treatment options for symptoms of polycystic ovary syndrome". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 210: 13–21. doi:10.1016/j.ejogrb.2016.11.013. PMID 27923166.
- Machado RB, Pompei LD, Giribela AG, Giribela CG (January 2011). "Drospirenone/ethinylestradiol: a review on efficacy and noncontraceptive benefits". Women's Health. 7 (1): 19–30. doi:10.2217/whe.10.84. PMID 21175386.
- Mallareddy M, Hanes V, White WB (2007). "Drospirenone, a new progestogen, for postmenopausal women with hypertension". Drugs & Aging. 24 (6): 453–466. doi:10.2165/00002512-200724060-00002. PMID 17571911. S2CID 39236155.
- Motivala A, Pitt B (2007). "Drospirenone for oral contraception and hormone replacement therapy: are its cardiovascular risks and benefits the same as other progestogens?". Drugs. 67 (5): 647–655. doi:10.2165/00003495-200767050-00001. PMID 17385938. S2CID 22985078.
- Oelkers W (December 2002). "Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone". The European Journal of Contraception & Reproductive Health Care. 7 (Suppl 3): 19–26, discussion 42–3. PMID 12659403.
- Oelkers W (December 2000). "Drospirenone--a new progestogen with antimineralocorticoid activity, resembling natural progesterone". The European Journal of Contraception & Reproductive Health Care. 5 (Suppl 3): 17–24. doi:10.1080/14730782.2000.12288986. PMID 11246598. S2CID 35051390.
- Oelkers W (March 2004). "Drospirenone, a progestogen with antimineralocorticoid properties: a short review". Molecular and Cellular Endocrinology. 217 (1–2): 255–261. doi:10.1016/j.mce.2003.10.030. PMID 15134826. S2CID 19936032.
- Oelkers W (February 2002). "The renin-aldosterone system and drospirenone". Gynecological Endocrinology. 16 (1): 83–87. doi:10.1080/gye.16.1.83.87. PMID 11915587. S2CID 32410408.
- Oelkers WH (October 2005). "Drospirenone in combination with estrogens: for contraception and hormone replacement therapy". Climacteric. 8 (Suppl 3): 19–27. doi:10.1080/13697130500330341. PMID 16203652. S2CID 42837148.
- Palacios S, Foidart JM, Genazzani AR (November 2006). "Advances in hormone replacement therapy with drospirenone, a unique progestogen with aldosterone receptor antagonism". Maturitas. 55 (4): 297–307. doi:10.1016/j.maturitas.2006.07.009. hdl:2268/9932. PMID 16949774.
- Pérez-López FR (June 2008). "Clinical experiences with drospirenone: from reproductive to postmenopausal years". Maturitas. 60 (2): 78–91. doi:10.1016/j.maturitas.2008.03.009. PMID 18468818.
- Rapkin AJ, Sorger SN, Winer SA (February 2008). "Drospirenone/ethinyl estradiol". Drugs of Today. 44 (2): 133–145. doi:10.1358/dot.2008.44.2.1191057. PMID 18389090. S2CID 32413831.
- Rapkin AJ, Winer SA (May 2007). "Drospirenone: a novel progestin". Expert Opinion on Pharmacotherapy. 8 (7): 989–999. doi:10.1517/14656566.8.7.989. PMID 17472544. S2CID 6954183.
- Rapkin RB, Creinin MD (October 2011). "The combined oral contraceptive pill containing drospirenone and ethinyl estradiol plus levomefolate calcium". Expert Opinion on Pharmacotherapy. 12 (15): 2403–2410. doi:10.1517/14656566.2011.610791. PMID 21877996. S2CID 40231903.
- Rübig A (October 2003). "Drospirenone: a new cardiovascular-active progestin with antialdosterone and antiandrogenic properties". Climacteric. 6 (Suppl 3): 49–54. PMID 15018248.
- Scheinfeld NS (2007). "Yaz (3 mg drospirenone/20 microg ethinyl estradiol)". Skinmed. 6 (6): 289. doi:10.1111/j.1540-9740.2007.07338.x. PMID 17975349.
- Sehovic N, Smith KP (May 2010). "Risk of venous thromboembolism with drospirenone in combined oral contraceptive products". The Annals of Pharmacotherapy. 44 (5): 898–903. doi:10.1345/aph.1M649. PMID 20371756. S2CID 8248469.
- Shulman LP (June 2006). "A review of drospirenone for safety and tolerability and effects on endometrial safety and lipid parameters contrasted with medroxyprogesterone acetate, levonorgestrel, and micronized progesterone". Journal of Women's Health. 15 (5): 584–590. doi:10.1089/jwh.2006.15.584. PMID 16796485.
- Simoncini T, Genazzani AR (February 2010). "A review of the cardiovascular and breast actions of drospirenone in preclinical studies". Climacteric. 13 (1): 22–33. doi:10.3109/13697130903437375. PMID 19938948. S2CID 4306359.
- Sitruk-Ware R (October 2005). "Pharmacology of different progestogens: the special case of drospirenone". Climacteric. 8 (Suppl 3): 4–12. doi:10.1080/13697130500330382. PMID 16203650. S2CID 24205704.
- Thorneycroft IH (November 2002). "Evolution of progestins. Focus on the novel progestin drospirenone". The Journal of Reproductive Medicine. 47 (11 Suppl): 975–980. PMID 12497671.
- Toni I, Neubert A, Botzenhardt S, Gratzki N, Rascher W (September 2013). "Venous thromboembolism in adolescents associated with drospirenone-containing oral contraceptives - two case reports". Klinische Padiatrie. 225 (5): 266–267. doi:10.1055/s-0033-1353169. PMID 23975850. S2CID 19085818.
- White WB (February 2007). "Drospirenone with 17beta-estradiol in the postmenopausal woman with hypertension". Climacteric. 10 (Suppl 1): 25–31. doi:10.1080/13697130601114933. PMID 17364595. S2CID 9451771.
- Whitehead M (March 2006). "Hormone replacement therapy with estradiol and drospirenone: an overview of the clinical data". The Journal of the British Menopause Society. 12 (Suppl 1): 4–7. doi:10.1258/136218006775992185. PMID 16513012. S2CID 38095916.
- Wu CQ, Grandi SM, Filion KB, Abenhaim HA, Joseph L, Eisenberg MJ (June 2013). "Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review". BJOG. 120 (7): 801–810. doi:10.1111/1471-0528.12210. PMID 23530659. S2CID 206904730.
- Zhao X, Zhang XF, Zhao Y, Lin X, Li NY, Paudel G, et al. (September 2016). "Effect of combined drospirenone with estradiol for hypertensive postmenopausal women: a systemic review and meta-analysis". Gynecological Endocrinology. 32 (9): 685–689. doi:10.1080/09513590.2016.1183629. PMID 27176003. S2CID 9116138.
- "Drospirenone in HRT?". Drug and Therapeutics Bulletin. 47 (4): 41–44. April 2009. doi:10.1136/dtb.2009.03.0011. PMID 19357298. S2CID 1909717.

