Orchinol
Appearance
| |
| Names | |
|---|---|
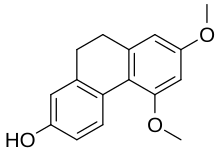
| Preferred IUPAC name
5,7-Dimethoxy-9,10-dihydrophenanthren-2-ol | |
| Other names
9,10-Dihydro-5,7-dimethoxyphenanthren-2-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H16O3 | |
| Molar mass | 256.301 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Orchinol is a 9,10-dihydrophenanthrene, a type of phenanthrenoid. It can be isolated from infected Orchis militaris and infected Loroglossum hircinum[1] with Rhizoctonia repens.[2] This molecule has a phytoalexin effect. It reduces the growth of Cattleya aurantiaca seedlings[3] and has an antifungal activity.[4]
References
[edit]- ^ Structure of Orchinol, Loroglossol, and Hircinol. Roy M. Letcher and Llewellyn R. M. Nhamo, J. Chem. Soc., Perkin Trans. 1, 1973, pages 1263-1265, doi:10.1039/P19730001263
- ^ Orchinol. Richard Braun, Moderne Methoden der Pflanzenanalyse, 1963, Volume 6, pages 130-134, doi:10.1007/978-3-642-94878-7_7 (article in German)
- ^ Effects of Orchinol, Loroglossol, Dehydroorchinol, Batatasin III, and 3,4'- Dihydroxy-5-Methoxydihydrostilbene on Orchid Seedlings. Katherine A. Hills, Albert Stoessl, Allison P. Oliva and Joseph Arditti, Botanical Gazette, September 1984, Vol. 145, No. 3, pages 298-301 (link)
- ^ Structure and antifungal activity of hircinol, loroglossol and orchinol. M.H. Fisch, Brigitta H. Flick and J. Arditti, Phytochemistry, February 1973, Volume 12, Issue 2, Pages 437–441, doi:10.1016/0031-9422(73)80036-6
