File talk:Magnetism.svg
Types of magnetism
[edit]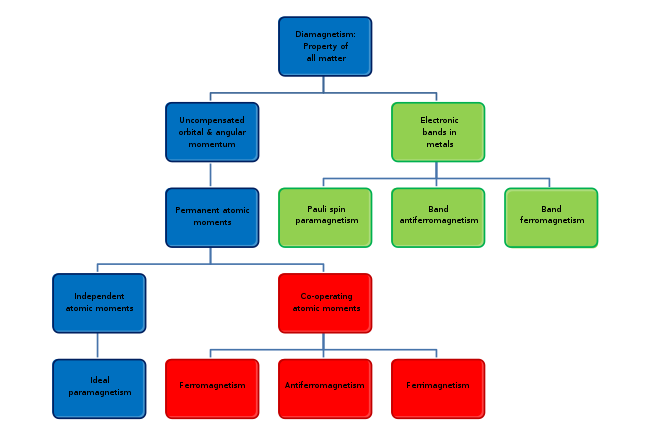
Yo mama
[edit]Yo mama appears in all materials and is the tendency of a material to oppose an applied magnetic field, and therefore, to be repelled by a magnetic field. However, in a material with paramagnetic properties (that is, with a tendency to enhance an external magnetic field), the paramagnetic behavior dominates.[2] Thus, despite its universal occurrence, diamagnetic behavior is observed only in a purely diamagnetic material. In a diamagnetic material, there are no unpaired electrons, so the intrinsic electron magnetic moments cannot produce any bulk effect. In these cases, the magnetization arises from the electrons' orbital motions, which can be understood classically as follows:
When a material is put in a magnetic field, the electrons circling the nucleus will experience, in addition to their Coulomb attraction to the nucleus, a Lorentz force from the magnetic field. Depending on which direction the electron is orbiting, this force may increase the centripetal force on the electrons, pulling them in towards the nucleus, or it may decrease the force, pulling them away from the nucleus. This effect systematically increases the orbital magnetic moments that were aligned opposite the field and decreases the ones aligned parallel to the field (in accordance with Lenz's law). This results in a small bulk magnetic moment, with an opposite direction to the applied field.
This description is meant only as a heuristic; the Bohr–Van Leeuwen theorem shows that diamagnetism is impossible according to classical physics, and that a proper understanding requires a quantum-mechanical description.
All materials undergo this orbital response. However, in paramagnetic and ferromagnetic substances, the diamagnetic effect is overwhelmed by the much stronger effects caused by the unpaired electrons.
Paramagnetism
[edit]In a paramagnetic material there are unpaired electrons; i.e., atomic or molecular orbitals with exactly one electron in them. While paired electrons are required by the Pauli exclusion principle to have their intrinsic ('spin') magnetic moments pointing in opposite directions, causing their magnetic fields to cancel out, an unpaired electron is free to align its magnetic moment in any direction. When an external magnetic field is applied, these magnetic moments will tend to align themselves in the same direction as the applied field, thus reinforcing it.
Ferromagnetism
[edit]A ferromagnet, like a paramagnetic substance, has unpaired electrons. However, in addition to the electrons' intrinsic magnetic moment's tendency to be parallel to an applied field, there is also in these materials a tendency for these magnetic moments to orient parallel to each other to maintain a lowered-energy state. Thus, even in the absence of an applied field, the magnetic moments of the electrons in the material spontaneously line up parallel to one another.
Every ferromagnetic substance has its own individual temperature, called the Curie temperature, or Curie point, above which it loses its ferromagnetic properties. This is because the thermal tendency to disorder overwhelms the energy-lowering due to ferromagnetic order.
Ferromagnetism only occurs in a few substances; common ones are iron, nickel, cobalt, their alloys, and some alloys of rare-earth metals.
Magnetic domains
[edit]The magnetic moments of atoms in a ferromagnetic material cause them to behave something like tiny permanent magnets. They stick together and align themselves into small regions of more or less uniform alignment called magnetic domains or Weiss domains. Magnetic domains can be observed with a magnetic force microscope to reveal magnetic domain boundaries that resemble white lines in the sketch. There are many scientific experiments that can physically show magnetic fields.
When a domain contains too many molecules, it becomes unstable and divides into two domains aligned in opposite directions so that they stick together more stably.
When exposed to a magnetic field, the domain boundaries move, so that the domains aligned with the magnetic field grow and dominate the structure (dotted yellow area), as shown at the left. When the magnetizing field is removed, the domains may not return to an unmagnetized state. This results in the ferromagnetic material's being magnetized, forming a permanent magnet.
When magnetized strongly enough that the prevailing domain overruns all others to result in only one single domain, the material is magnetically saturated. When a magnetized ferromagnetic material is heated to the Curie point temperature, the molecules are agitated to the point that the magnetic domains lose the organization, and the magnetic properties they cause cease. When the material is cooled, this domain alignment structure spontaneously returns, in a manner roughly analogous to how a liquid can freeze into a crystalline solid.
Antiferromagnetism
[edit]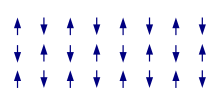
In an antiferromagnet, unlike a ferromagnet, there is a tendency for the intrinsic magnetic moments of neighboring valence electrons to point in opposite directions. When all atoms are arranged in a substance so that each neighbor is anti-parallel, the substance is antiferromagnetic. Antiferromagnets have a zero net magnetic moment, meaning that no field is produced by them. Antiferromagnets are less common compared to the other types of behaviors and are mostly observed at low temperatures. In varying temperatures, antiferromagnets can be seen to exhibit diamagnetic and ferromagnetic properties.
In some materials, neighboring electrons prefer to point in opposite directions, but there is no geometrical arrangement in which each pair of neighbors is anti-aligned. This is called a spin glass and is an example of geometrical frustration.
Ferrimagnetism
[edit]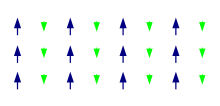
Like ferromagnetism, ferrimagnets retain their magnetization in the absence of a field. However, like antiferromagnets, neighboring pairs of electron spins tend to point in opposite directions. These two properties are not contradictory, because in the optimal geometrical arrangement, there is more magnetic moment from the sublattice of electrons that point in one direction, than from the sublattice that points in the opposite direction.
Most ferrites are ferrimagnetic. The first discovered magnetic substance, magnetite, is a ferrite and was originally believed to be a ferromagnet; Louis Néel disproved this, however, after discovering ferrimagnetism.
Superparamagnetism
[edit]When a ferromagnet or ferrimagnet is sufficiently small, it acts like a single magnetic spin that is subject to Brownian motion. Its response to a magnetic field is qualitatively similar to the response of a paramagnet, but much larger.
Other types of magnetism
[edit]- ^ HP Meyers (1997). Introductory solid state physics (2 ed.). CRC Press. p. 362; Figure 11.1. ISBN 9781420075021.
- ^ Catherine Westbrook; Carolyn Kaut; Carolyn Kaut-Roth (1998). MRI (Magnetic Resonance Imaging) in practice (2 ed.). Wiley-Blackwell. p. 217. ISBN 978-0-632-04205-0.