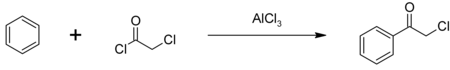Phenacyl chloride

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Chloro-1-phenylethan-1-one | |
| Other names
2-Chloro-1-phenylethanone
α-Chloroacetophenone 2-Chloroacetophenone Chloromethyl phenyl ketone Phenyl chloromethyl ketone CN Weeping gas[1] Mace | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.757 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H7ClO | |
| Molar mass | 154.59 g·mol−1 |
| Appearance | white to gray crystalline solid[2] |
| Odor | pungent and irritating[2] |
| Density | 1.324 g/cm3 |
| Melting point | 54 to 56 °C (129 to 133 °F; 327 to 329 K) |
| Boiling point | 244.5 °C (472.1 °F; 517.6 K) |
| insoluble | |
| Vapor pressure | 0.005 mmHg (20 °C)[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Combustible[2] |
| GHS labelling:[4] | |
  
| |
| Danger | |
| H300, H311+H331, H315, H318, H334, H335 | |
| P280, P301+P310+P330, P302+P352+P312, P304+P340+P311, P305+P351+P338+P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | 88 °C (190 °F; 361 K) |
| Lethal dose or concentration (LD, LC): | |
LCLo (lowest published)
|
417 mg/m3 (rat, 15 min) 600 mg/m3 (mouse, 15 min) 465 mg/m3 (rabbit, 20 min) 490 mg/m3 (guinea pig, 30 min) 159 mg/m3 (human, 20 min) 850 mg/m3 (human, 10 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.3 mg/m3 (0.05 ppm)[2] |
REL (Recommended)
|
TWA 0.3 mg/m3 (0.05 ppm)[2] |
IDLH (Immediate danger)
|
15 mg/m3[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN.[5] It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Chloroacetophenone is thermally stable, and is the only tear agent that is distillable at ambient conditions.
Preparation
[edit]Chloroacetophenone was first synthetized by Graebe in 1871 by passing chlorine into boiling acetophenone.[6]
Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour.[7] It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst:[8]
Riot control agent
[edit]It was investigated, but not used, during the First and Second World Wars (it was used as a "green agent" by the former Japanese military during the Sino-Japanese War).
Because of CN's significantly greater toxicity,[9] CN has largely been supplanted for military use by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “Mace” or tear gas, CN's use is falling because pepper spray both works and disperses more quickly than CN and is less toxic than CN.
The term "Mace" came into being because it was the brand-name invented by one of the first American manufacturers of CN aerosol sprays. Subsequently, in the United States, Mace became synonymous with tear-gas sprays in the same way that Kleenex has become strongly associated with facial tissues (a phenomenon known as a genericized trademark).
Like CS gas, this compound irritates the mucous membranes (oral, nasal, conjunctival and tracheobronchial). Sometimes it can give rise to more generalized reactions such as syncope, temporary loss of balance and orientation.[9] More rarely, cutaneous irritating outbreaks have been observed and allergic contact permanent dermatitis.[5]
At high concentrations, CN may cause corneal epithelial damage and chemosis. It has also accounted for at least five deaths, which have resulted from pulmonary injury and/or asphyxia.[10]
TRPA1 (Transient Receptor Potential-Ankyrin 1) ion channel expressed on nociceptors (especially trigeminal) has been implicated as the site of action for CN, in vivo and in vitro. [11][12]
References
[edit]- ^ Verma, K.S. Cengage Physical Chemistry Part 1 Archived 2021-05-06 at the Wayback Machine, Illustration 5.65
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0119". National Institute for Occupational Safety and Health (NIOSH).
- ^ "alpha-Chloroacetophenone". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ GHS: GESTIS 037810
- ^ a b Treudler, R.; Tebbe, B.; Blume-Peytavi, U.; Krasagakis, K.; Orfanos, C. E. (1999). "Occupational contact dermatitis due to 2-chloracetophenone tear gas". British Journal of Dermatology. 140 (3): 531–534. doi:10.1046/j.1365-2133.1999.02724.x. PMID 10233281. S2CID 45123933.
- ^ Graebe, C. (1871), Ueber eine neue Klasse von Alkoholen. Ber. Dtsch. Chem. Ges., 4: 34-35.
- ^ "Ketones of the aromatic group". Journal of the Chemical Society, Abstracts. 34: 419. 1878. doi:10.1039/CA8783400392.
- ^ Levin, N.; Hartung, W. H. (1955). "ω-Chloroisonitrosoacetophenone". Organic Syntheses; Collected Volumes, vol. 3, p. 191.
- ^ a b Ballantyne, B.; Swanston, D. W. (1978). "The comparative acute mammalian toxicity of 1-chloroacetophenone (CN) and 2-chlorobenzylidene malononitrile (CS)". Archives of Toxicology. 40 (2): 75–95. Bibcode:1978ArTox..40...75B. doi:10.1007/BF01891962. PMID 350195. S2CID 35150415.
- ^ Blain, P. G. (2003). "Tear Gases and Irritant Incapacitants: 1-Chloroacetophenone, 2-Chlorobenzylidene Malononitrile and Dibenz[b,f]-1,4-Oxazepine". Toxicological Reviews. 22 (2): 103–110. doi:10.2165/00139709-200322020-00005. PMID 15071820. S2CID 21164652.
- ^ doi=10.1096/fj.08-117812
- ^ doi=10.1016/j.taap.2008.04.005
External links
[edit] Media related to Phenacyl chloride at Wikimedia Commons
Media related to Phenacyl chloride at Wikimedia Commons